Deck 2: Lifes Chemical Basis
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/66
Play
Full screen (f)
Deck 2: Lifes Chemical Basis
1
The atom found in the greatest amount in the human body is
A)hydrogen.
B)carbon.
C)nitrogen.
D)oxygen.
E)phosphorus.
A)hydrogen.
B)carbon.
C)nitrogen.
D)oxygen.
E)phosphorus.
A
2
The atomic number is the number of
A)neutrons and protons.
B)neutrons and electrons.
C)protons and electrons.
D)protons only.
E)neutrons only.
A)neutrons and protons.
B)neutrons and electrons.
C)protons and electrons.
D)protons only.
E)neutrons only.
D
3
The periodic table of the elements was devised by
A)Henri Becquerel.
B)Demitri Mendeleev.
C)Melvin Calvin.
D)Marie Curie.
E)Becquerel and Mendeleev.
A)Henri Becquerel.
B)Demitri Mendeleev.
C)Melvin Calvin.
D)Marie Curie.
E)Becquerel and Mendeleev.
B
4
Which components of an atom do not have a charge? I.electrons
II)protons
III)neutrons
A)I only
B)II only
C)III only
D)I and II
E)II and III
II)protons
III)neutrons
A)I only
B)II only
C)III only
D)I and II
E)II and III

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
5
The atomic number refers to the
A)mass of an atom.
B)number of protons in an atom.
C)number of both protons and neutrons in an atom.
D)number of neutrons in an atom.
E)number of electrons in an atom.
A)mass of an atom.
B)number of protons in an atom.
C)number of both protons and neutrons in an atom.
D)number of neutrons in an atom.
E)number of electrons in an atom.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
6
Whether one atom will bond with another depends on the element and the number and arrangement of its
A)protons.
B)neutrons.
C)electrons.
D)neutrinos.
E)pions.
A)protons.
B)neutrons.
C)electrons.
D)neutrinos.
E)pions.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
7
The subatomic particle(s) with no charge is(are)
A)the neutron.
B)the proton.
C)the electron.
D)both the neutron and proton.
E)both the proton and electron.
A)the neutron.
B)the proton.
C)the electron.
D)both the neutron and proton.
E)both the proton and electron.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
8
The subatomic particle(s) with a positive charge is(are)
A)the neutron.
B)the proton. c the electron.
D)both the neutron and proton.
E)both the proton and electron.
A)the neutron.
B)the proton. c the electron.
D)both the neutron and proton.
E)both the proton and electron.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
9
The nucleus of an atom contains
A)neutrons and protons.
B)neutrons and electrons.
C)protons and electrons.
D)protons only.
E)neutrons only.
A)neutrons and protons.

B)neutrons and electrons.
C)protons and electrons.
D)protons only.
E)neutrons only.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
10
Which components of an atom have negative charges? I.electrons
II)protons
III)neutrons
A)I only
B)II only
C)III only
D)I and II
E)II and III
II)protons
III)neutrons
A)I only
B)II only
C)III only
D)I and II
E)II and III

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
11
All atoms of an element have the same number of
A)ions.
B)protons.
C)neutrons.
D)electrons.
E)protons and neutrons.
A)ions.
B)protons.
C)neutrons.
D)electrons.
E)protons and neutrons.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
12
The collection of elements that make up the human body are worth approximately
A)46 thousand dollars.
B)30 million dollars.
C)118 dollars.
D)5 thousand dollars.
E)none of these.
A)46 thousand dollars.
B)30 million dollars.
C)118 dollars.
D)5 thousand dollars.
E)none of these.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
13
Toxic elements such as mercury and arsenic are found in the human body because
A)of contamination from the environment.
B)trace amounts of these elements have vital biological functions.
C)they are needed to kill bacteria.
D)they may be ingested with food but inactivated by cells.
E)in small amounts they are biologically inactive and tolerated by cells.
A)of contamination from the environment.
B)trace amounts of these elements have vital biological functions.
C)they are needed to kill bacteria.
D)they may be ingested with food but inactivated by cells.
E)in small amounts they are biologically inactive and tolerated by cells.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is false concerning the atom in the figure?
A)The number of protons and the number of electrons are equal.
B)It has an atomic mass of 4.
C)Electrons are moving around the nucleus.
D)It has an atomic number of 2.
E)The number of electrons exceeds the number of protons.
A)The number of protons and the number of electrons are equal.
B)It has an atomic mass of 4.
C)Electrons are moving around the nucleus.
D)It has an atomic number of 2.
E)The number of electrons exceeds the number of protons.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following historical figures came up with the idea of the periodic table?
A)Demitri Medeleev
B)Niels Bohr
C)Louis Pateur
D)Robert Koch
E)None of these
A)Demitri Medeleev
B)Niels Bohr
C)Louis Pateur
D)Robert Koch
E)None of these

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
16
Which is the smallest unit of an element that retains the properties of the element?
A)atom
B)compound
C)ion
D)molecule
E)mixture
A)atom
B)compound
C)ion
D)molecule
E)mixture

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
17
Which is NOT an element?
A)water
B)oxygen
C)carbon
D)chlorine
E)hydrogen
A)water
B)oxygen
C)carbon
D)chlorine
E)hydrogen

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
18
The atomic mass (mass number)of an atom is determined by the combined masses of its
A)neutrons and protons.
B)neutrons and electrons.
C)protons and electrons.
D)protons, neutrons, and electrons.
E)neutrons, nucleus, and electrons.
A)neutrons and protons.
B)neutrons and electrons.
C)protons and electrons.
D)protons, neutrons, and electrons.
E)neutrons, nucleus, and electrons.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
19
Atoms of isotopes
A)are electrically unbalanced.
B)behave the same chemically and physically but differ biologically from other isotopes.
C)are the same physically and biologically but differ from other isotopes chemically.
D)have the same number of protons but a different number of neutrons.
E)are produced when atoms lose electrons.
A)are electrically unbalanced.
B)behave the same chemically and physically but differ biologically from other isotopes.
C)are the same physically and biologically but differ from other isotopes chemically.
D)have the same number of protons but a different number of neutrons.
E)are produced when atoms lose electrons.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
20
The subatomic particle(s) with a negative charge is(are)
A)the neutron.
B)the proton.
C)the electron.
D)both the neutron and proton.
E)both the proton and electron.
A)the neutron.
B)the proton.
C)the electron.
D)both the neutron and proton.
E)both the proton and electron.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
21
Which statement concerning radioisotope 14C is false?
A)It can be substituted for 12C in glucose and the body will still be able to use the compound.
B)It has a different number of protons than 12C.
C)It has more neutrons than 12C.
D)It behaves the same chemically as 12C.
E)It has six carbons and eight neutrons.
A)It can be substituted for 12C in glucose and the body will still be able to use the compound.
B)It has a different number of protons than 12C.
C)It has more neutrons than 12C.
D)It behaves the same chemically as 12C.
E)It has six carbons and eight neutrons.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
22
A molecule is
A)a combination of two or more atoms.
B)a mixture of atoms.
C)electrically charged.
D)a carrier of one or more extra neutrons.
E)none of these.
A)a combination of two or more atoms.
B)a mixture of atoms.
C)electrically charged.
D)a carrier of one or more extra neutrons.
E)none of these.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
23
Tracers are elements that
A)are used in minute amounts in plants.
B)can be monitored through biochemical reactions.
C)must be inert.
D)have an unbalanced electrical charge.
E)must have a stable nucleus.
A)are used in minute amounts in plants.
B)can be monitored through biochemical reactions.
C)must be inert.
D)have an unbalanced electrical charge.
E)must have a stable nucleus.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
24
Radioactive isotopes have
A)excess electrons.
B)excess protons.
C)excess neutrons.
D)insufficient neutrons.
E)insufficient protons.
A)excess electrons.
B)excess protons.
C)excess neutrons.
D)insufficient neutrons.
E)insufficient protons.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
25
Water is an example of a(n)
A)atom.
B)ion.
C)compound.
D)mixture.
E)element.
A)atom.
B)ion.
C)compound.
D)mixture.
E)element.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
26
Nitrogen has an atomic number of 7.How many hydrogen atoms are necessary to join with the nitrogen to form a stable compound?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is NOT accurate concerning ionization?
A)When one atom loses electrons, another must gain electrons.
B)When an atom loses an electron, it becomes negatively charged.
C)Ionic bonds form between ionized atoms.
D)In the compound NaCl, Na loses an electron to become positive.
E)In an ion, the number of protons and electrons is unequal.
A)When one atom loses electrons, another must gain electrons.
B)When an atom loses an electron, it becomes negatively charged.
C)Ionic bonds form between ionized atoms.
D)In the compound NaCl, Na loses an electron to become positive.
E)In an ion, the number of protons and electrons is unequal.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
28
Which includes the other four?
A)atoms
B)molecules
C)electrons
D)elements
E)protons
A)atoms
B)molecules
C)electrons
D)elements
E)protons

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
29
Magnesium has 12 protons.How many electrons are in its first energy level?
A)2
B)4
C)6
D)8
E)10
A)2
B)4
C)6
D)8
E)10

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
30
Which statement is NOT true?
A)Electrons closest to the nucleus are at the lowest energy level.
B)No more than two electrons can occupy a single orbital.
C)Electrons are unable to move out of the assigned orbital space.
D)The innermost orbital holds two electrons.
E)At the second energy level there are four possible orbitals with a total of eight electrons.
A)Electrons closest to the nucleus are at the lowest energy level.
B)No more than two electrons can occupy a single orbital.
C)Electrons are unable to move out of the assigned orbital space.
D)The innermost orbital holds two electrons.
E)At the second energy level there are four possible orbitals with a total of eight electrons.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
31
An atom that gains or loses electrons becomes
A)more stable.
B)an ion.
C)a molecule.
D)unable to form bonds.
E)radioactive.
A)more stable.
B)an ion.
C)a molecule.
D)unable to form bonds.
E)radioactive.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
32
Which is NOT a compound?
A)salt (NaCl)
B)a carbohydrate (contains C, H and O)
C)carbon (C)
D)a nucleotide (contains P, C, H, and O)
E)methane (CH4)
A)salt (NaCl)
B)a carbohydrate (contains C, H and O)
C)carbon (C)
D)a nucleotide (contains P, C, H, and O)
E)methane (CH4)

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
33
The radioactive decay of 14C produces
A)carbon 12.
B)carbon 13.
C)more carbon 14.
D)nitrogen 14.
E)oxygen 14.
A)carbon 12.
B)carbon 13.
C)more carbon 14.
D)nitrogen 14.
E)oxygen 14.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
34
By analogy, the orbitals and atomic nucleus may be said to most resemble
A)a merry-go-round.
B)a sundial.
C)a multilevel apartment building.
D)a nest of mixing bowls.
E)ripples in a pond.
A)a merry-go-round.
B)a sundial.
C)a multilevel apartment building.
D)a nest of mixing bowls.
E)ripples in a pond.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
35
Magnesium has 12 protons.How many electrons are in its third energy level?
A)2
B)4
C)6
D)8
E)10
A)2
B)4
C)6
D)8
E)10

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
36
__________ molecule(s) can be detected as it (they) pass(es) through the human body and is (are) used for diagnosis.
A)A decay
B)A tracer
C)An electron
D)An atomic
E)Both the proton and electron
A)A decay
B)A tracer
C)An electron
D)An atomic
E)Both the proton and electron

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements is NOT true?
A)All isotopes of an element have the same number of electrons.
B)All isotopes of an element have the same number of protons.
C)All isotopes of an element have the same number of neutrons.
D)We refer to isotopes by mass number.
E)12C and 13C are isotopes.
A)All isotopes of an element have the same number of electrons.
B)All isotopes of an element have the same number of protons.
C)All isotopes of an element have the same number of neutrons.
D)We refer to isotopes by mass number.
E)12C and 13C are isotopes.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
38
In the chemical shorthand 14C, the 14 represents the number of
A)excess neutrons.
B)protons plus neutrons.
C)electrons.
D)protons plus electrons.
E)radioactive particles.
A)excess neutrons.
B)protons plus neutrons.
C)electrons.
D)protons plus electrons.
E)radioactive particles.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
39
Magnesium has 12 protons.How many electrons are in its second energy level?
A)2
B)4
C)6
D)8
E)10
A)2
B)4
C)6
D)8
E)10

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
40
Which statement is false?
A)A molecule is made of at least two atoms.
B)Compounds are made of elements.
C)Two atoms of oxygen make a molecule of oxygen.
D)Proportions of elements in compounds vary according to their source in nature.
E)Elements are found in compounds and molecules.
A)A molecule is made of at least two atoms.
B)Compounds are made of elements.
C)Two atoms of oxygen make a molecule of oxygen.
D)Proportions of elements in compounds vary according to their source in nature.
E)Elements are found in compounds and molecules.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is(are) true of water?
A)It forms spheres of hydration around charged substances and can form hydrogen bonds with many substances.
B)It has a high heat-containing property.
C)It has cohesive properties.
D)It is a liquid at room temperature.
E)It is all of the above.
A)It forms spheres of hydration around charged substances and can form hydrogen bonds with many substances.
B)It has a high heat-containing property.
C)It has cohesive properties.
D)It is a liquid at room temperature.
E)It is all of the above.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
42
Water is important to the interactions of biological molecules because it
A)promotes hydrophobic and hydrophilic interactions.
B)stabilizes temperature.
C)is an excellent solvent for polar and ionic substances.
D)has strong cohesive properties.
E)is all of the above.
A)promotes hydrophobic and hydrophilic interactions.
B)stabilizes temperature.
C)is an excellent solvent for polar and ionic substances.
D)has strong cohesive properties.
E)is all of the above.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
43
The measurement of the temperature of a substance is directly related to
A)the nature of its chemical bonds.
B)its reactivity.
C)its thermal conductivity.
D)the motion of its molecules.
E)the mass of its molecules.
A)the nature of its chemical bonds.
B)its reactivity.
C)its thermal conductivity.
D)the motion of its molecules.
E)the mass of its molecules.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements is false?
A)Ice is denser than liquid water.
B)All living organisms require water.
C)Water has cohesive properties.
D)Water is a liquid at room temperature.
E)All of the above are false.
A)Ice is denser than liquid water.
B)All living organisms require water.
C)Water has cohesive properties.
D)Water is a liquid at room temperature.
E)All of the above are false.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
45
Electrons are shared in bonds that are
A)covalent.
B)polar.
C)nonpolar.
D)covalent, polar, or nonpolar.
E)covalent, but not polar or nonpolar.
A)covalent.
B)polar.
C)nonpolar.
D)covalent, polar, or nonpolar.
E)covalent, but not polar or nonpolar.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
46
The solvent, cohesive, and temperature stabilization properties of water are due to its
A)ability to promote hydrophilic interactions.
B)ionic bonds.
C)hydrogen bonds.
D)ability to promote hydrophobic interactions.
E)nonpolar nature.
A)ability to promote hydrophilic interactions.
B)ionic bonds.
C)hydrogen bonds.
D)ability to promote hydrophobic interactions.
E)nonpolar nature.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
47
The column of water extending in tubes from plant roots to leaves is maintained by
A)cohesion among water molecules.
B)ionic bonds.
C)covalent bonds.
D)hydrophobic interactions.
E)hydrophilic interactions.
A)cohesion among water molecules.
B)ionic bonds.
C)covalent bonds.
D)hydrophobic interactions.
E)hydrophilic interactions.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is true of water?
A)The oxygen end is slightly electropositive.
B)Hydrogen bonds hold water molecules together.
C)Water covers about one-half of the earth's surface.
D)It participates in hydrophobic interactions with polar molecules.
E)Its solvent properties are greatest with nonpolar molecules.
A)The oxygen end is slightly electropositive.
B)Hydrogen bonds hold water molecules together.
C)Water covers about one-half of the earth's surface.
D)It participates in hydrophobic interactions with polar molecules.
E)Its solvent properties are greatest with nonpolar molecules.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
49
The most likely reason that glucose dissolves in water is that it is
A)an ionic compound.
B)a polysaccharide.
C)polar and forms many hydrogen bonds with the water molecules.
D)a very unstable molecule.
E)highly nonpolar.
A)an ionic compound.
B)a polysaccharide.
C)polar and forms many hydrogen bonds with the water molecules.
D)a very unstable molecule.
E)highly nonpolar.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
50
The bond in table salt (NaCl) is
A)polar.
B)ionic.
C)covalent.
D)double.
E)nonpolar.
A)polar.
B)ionic.
C)covalent.
D)double.
E)nonpolar.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
51
In __________ bonds, both atoms exert the same pull on shared electrons.
A)nonpolar covalent
B)polar covalent
C)double covalent
D)triple covalent
E)coordinate covalent
A)nonpolar covalent
B)polar covalent
C)double covalent
D)triple covalent
E)coordinate covalent

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
52
Sodium chloride (NaCl) in water can be described by any EXCEPT which of the following?
A)Na+ and Cl- form
B)a solute
C)ionized
D)forms a hydrophobic interaction
E)dissolved
A)Na+ and Cl- form
B)a solute
C)ionized
D)forms a hydrophobic interaction
E)dissolved

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is NOT true of hydrogen bonds?
A)They are quite weak.
B)The hydrogen is slightly positive.
C)They are common in macromolecules.
D)They are difficult to form and break.
E)They always involve hydrogen.
A)They are quite weak.
B)The hydrogen is slightly positive.
C)They are common in macromolecules.
D)They are difficult to form and break.
E)They always involve hydrogen.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
54
Oxygen, with an atomic number of 8, has __________ electrons in the first energy level and __________ electrons in the second energy level.
A)1; 7
B)2; 6
C)3; 5
D)4; 4
E)5; 3
A)1; 7
B)2; 6
C)3; 5
D)4; 4
E)5; 3

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
55
Which of these statements is false concerning covalent bonds?
A)Atoms share electrons.
B)Molecules may possess many covalent bonds.
C)Water contains polar covalent bonds.
D)Covalent bonds may be "double bonds."
E)In polar covalent bonds, electrons are shared equally.
A)Atoms share electrons.
B)Molecules may possess many covalent bonds.
C)Water contains polar covalent bonds.
D)Covalent bonds may be "double bonds."
E)In polar covalent bonds, electrons are shared equally.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
56
A hydrogen bond is a(n)
A)sharing of a pair of electrons between a hydrogen and an oxygen nucleus.
B)sharing of a pair of electrons between a hydrogen nucleus and either an oxygen or a nitrogen nucleus.
C)attractive force between a hydrogen atom and either an oxygen or a nitrogen atom that are in other molecules or within the same molecule.
D)covalent bond between two hydrogen atoms.
E)covalent bond between a hydrogen atom and either an oxygen atom or a nitrogen atom.
A)sharing of a pair of electrons between a hydrogen and an oxygen nucleus.
B)sharing of a pair of electrons between a hydrogen nucleus and either an oxygen or a nitrogen nucleus.
C)attractive force between a hydrogen atom and either an oxygen or a nitrogen atom that are in other molecules or within the same molecule.
D)covalent bond between two hydrogen atoms.
E)covalent bond between a hydrogen atom and either an oxygen atom or a nitrogen atom.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
57
The dots in the figure represent a(n) 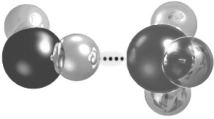
A)covalent bond.
B)ionic bond.
C)hydrogen bond.
D)polar covalent bond.
E)hydrophobic interaction.

A)covalent bond.
B)ionic bond.
C)hydrogen bond.
D)polar covalent bond.
E)hydrophobic interaction.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
58
Hydrophobic interactions are exhibited with
A)ions.
B)nonpolar molecules.
C)hydration ions.
D)polar molecules.
E)none of these.
A)ions.
B)nonpolar molecules.
C)hydration ions.
D)polar molecules.
E)none of these.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
59
Hydrophobic molecules are __________ water.
A)attracted to
B)absorbed by
C)repelled by
D)dissolved by
E)polarized by
A)attracted to
B)absorbed by
C)repelled by
D)dissolved by
E)polarized by

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
60
The oil globules that result when a water and oil mixture is shaken are due to a(n) __________ interaction.
A)acidic
B)basic
C)hydrophilic
D)hydrophobic
E)ionic
A)acidic
B)basic
C)hydrophilic
D)hydrophobic
E)ionic

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is NOT true?
A)Acids donate hydrogen ions.
B)In a neutral solution, the amounts of hydrogen and hydroxyl ions are equal.
C)Salts have no function in cells.
D)Bases accept hydrogen ions..
E).7.0 represents a neutral pH.
A)Acids donate hydrogen ions.
B)In a neutral solution, the amounts of hydrogen and hydroxyl ions are equal.
C)Salts have no function in cells.
D)Bases accept hydrogen ions..
E).7.0 represents a neutral pH.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
62
Most of the body's internal environment is at a pH between
A)6.8 and 7.2
B)7.0 and 7.2
C)6.5 and 7.5
D)7.3 and 7.5
E)7.5 and 8.0
A)6.8 and 7.2
B)7.0 and 7.2
C)6.5 and 7.5
D)7.3 and 7.5
E)7.5 and 8.0

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
63
A pH of 10 is how many times as basic as a pH of 7?
A)2
B)3
C)10
D)100
E)1,000
A)2
B)3
C)10
D)100
E)1,000

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
64
A salt will dissolve in water to form
A)acids.
B)hydrogen bonds.
C)ions other than H+ and OH-.
D)bases.
E)buffers.
A)acids.
B)hydrogen bonds.
C)ions other than H+ and OH-.
D)bases.
E)buffers.

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
65
A solution with a pH of 8 has how many times fewer hydrogen ions than a solution with a pH of 6?
A)2
B)4
C)10
D)100
E)1,000
A)2
B)4
C)10
D)100
E)1,000

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck
66
"Acidic" is an appropriate description for all EXCEPT which one of the following?
A)excess hydrogen ions
B)the contents of the stomach
C)magnesium hydroxide
D)HCl
E)a pH less than 7
A)excess hydrogen ions
B)the contents of the stomach
C)magnesium hydroxide
D)HCl
E)a pH less than 7

Unlock Deck
Unlock for access to all 66 flashcards in this deck.
Unlock Deck
k this deck


