Deck 10: The Reactions of Organic Functional Groups in Biochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/87
Play
Full screen (f)
Deck 10: The Reactions of Organic Functional Groups in Biochemistry
1
What does [H] represent in the following reaction? ![<strong>What does [H] represent in the following reaction? </strong> A) the oxidizing agent B) the species that is oxidized C) the reducing agent D) the species that is protonated E) atomic hydrogen](https://storage.examlex.com/TB7342/11eb145e_bfbd_7d8e_8021_0bb4459680ee_TB7342_00.jpg)
A) the oxidizing agent
B) the species that is oxidized
C) the reducing agent
D) the species that is protonated
E) atomic hydrogen
![<strong>What does [H] represent in the following reaction? </strong> A) the oxidizing agent B) the species that is oxidized C) the reducing agent D) the species that is protonated E) atomic hydrogen](https://storage.examlex.com/TB7342/11eb145e_bfbd_7d8e_8021_0bb4459680ee_TB7342_00.jpg)
A) the oxidizing agent
B) the species that is oxidized
C) the reducing agent
D) the species that is protonated
E) atomic hydrogen
the reducing agent
2
Cellular respiration and the combustion of fuel in a car engine are similar in many ways, but they also differ. What is one way in which they are different?
A) Cellular respiration occurs in many steps and the combustion of fuel in a single step.
B) Cellular respiration is exothermic and the combustion of fuel is endothermic.
C) Cellular respiration is endothermic and the combustion of fuel is exothermic.
D) Cellular respiration is fast and the combustion of fuel is slow.
E) Cellular respiration requires oxygen and the combustion of fuel does not.
A) Cellular respiration occurs in many steps and the combustion of fuel in a single step.
B) Cellular respiration is exothermic and the combustion of fuel is endothermic.
C) Cellular respiration is endothermic and the combustion of fuel is exothermic.
D) Cellular respiration is fast and the combustion of fuel is slow.
E) Cellular respiration requires oxygen and the combustion of fuel does not.
Cellular respiration occurs in many steps and the combustion of fuel in a single step.
3
In the following reaction, the sulfate ion does not react. What is this ion called? 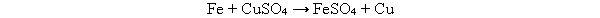
A) This ion has no special name.
B) It is a reactive ion.
C) It is a spectator ion.
D) It is an explosive ion.
E) It is a nonfunctional ion.
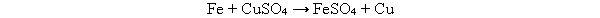
A) This ion has no special name.
B) It is a reactive ion.
C) It is a spectator ion.
D) It is an explosive ion.
E) It is a nonfunctional ion.
It is a spectator ion.
4
Which statement best describes what changes occur over the course of the following oxidation-reduction reaction? Fe + Cu2+ → Fe2+ + Cu
A) Two electrons are lost.
B) Iron changes into copper.
C) Iron transfers two electrons to copper.
D) Copper transfers two electrons to iron.
E) Two electrons are gained.
A) Two electrons are lost.
B) Iron changes into copper.
C) Iron transfers two electrons to copper.
D) Copper transfers two electrons to iron.
E) Two electrons are gained.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following characteristics is not shared by all combustion reactions?
A) They occur in multiple steps.
B) They require oxygen.
C) They release water and carbon dioxide.
D) They are exothermic.
E) They are a type of oxidation-reduction reaction.
A) They occur in multiple steps.
B) They require oxygen.
C) They release water and carbon dioxide.
D) They are exothermic.
E) They are a type of oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement best describes the following reaction? 
A) In this reaction, both iron and copper are reduced.
B) In this reaction, both iron and copper are oxidized.
C) In this reaction, iron is reduced and copper is oxidized.
D) In this reaction, iron is oxidized and copper is reduced.
E) This is not an oxidation-reduction reaction.

A) In this reaction, both iron and copper are reduced.
B) In this reaction, both iron and copper are oxidized.
C) In this reaction, iron is reduced and copper is oxidized.
D) In this reaction, iron is oxidized and copper is reduced.
E) This is not an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
7
The following reaction is an example of a(n) ___________ reaction. CH4 + 2O2 → CO2 + 2H2O
I. oxidation-reduction
II. acid-Base
III. combustion
A) I only
B) I and II only
C) III only
D) I and III only
E) I, II and III
I. oxidation-reduction
II. acid-Base
III. combustion
A) I only
B) I and II only
C) III only
D) I and III only
E) I, II and III

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is NOT an example of an oxidation-reduction reaction?
A)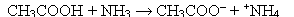
B)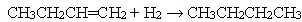
C)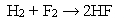
D)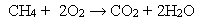
E)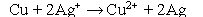
A)
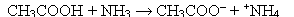
B)
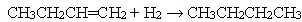
C)
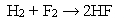
D)
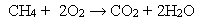
E)
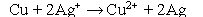

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following pairs of molecules will react similarly in the presence of an oxidant? 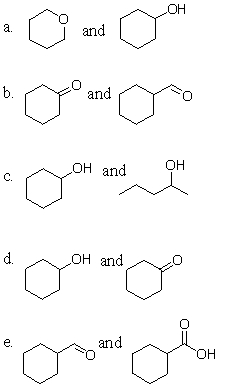
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement about vitamins is NOT true?
A) Water soluble vitamins are excreted in the urine.
B) Fat soluble vitamins are stored in fat.
C) Vitamin C is an example of a water soluble vitamin.
D) Water soluble vitamins should be replenished daily.
E) Fat soluble vitamins are readily excreted in the urine.
A) Water soluble vitamins are excreted in the urine.
B) Fat soluble vitamins are stored in fat.
C) Vitamin C is an example of a water soluble vitamin.
D) Water soluble vitamins should be replenished daily.
E) Fat soluble vitamins are readily excreted in the urine.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
11
Select the choice that correctly classifies vitamins by their solubility. 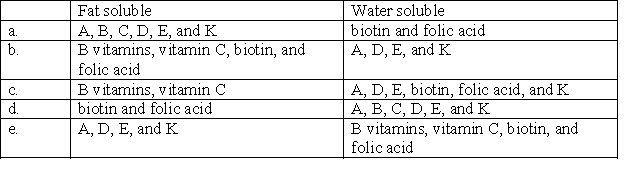
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
12
What does [O] represent in the following reaction? ![<strong>What does [O] represent in the following reaction? </strong> A) the oxidizing agent B) the species that is oxidized C) the reducing agent D) the species that is protonated E) atomic oxygen](https://storage.examlex.com/TB7342/11eb145e_bfbd_7d8d_8021_771730b1482d_TB7342_00.jpg)
A) the oxidizing agent
B) the species that is oxidized
C) the reducing agent
D) the species that is protonated
E) atomic oxygen
![<strong>What does [O] represent in the following reaction? </strong> A) the oxidizing agent B) the species that is oxidized C) the reducing agent D) the species that is protonated E) atomic oxygen](https://storage.examlex.com/TB7342/11eb145e_bfbd_7d8d_8021_771730b1482d_TB7342_00.jpg)
A) the oxidizing agent
B) the species that is oxidized
C) the reducing agent
D) the species that is protonated
E) atomic oxygen

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
13
Is the alkene oxidized or reduced during this reaction and how can you tell? 
A) Neither, this is not an oxidation-reduction reaction.
B) The alkene is both oxidized and reduced because this is an oxidation-reduction reaction.
C) The alkene is oxidized because it loses two hydrogens.
D) The alkene is reduced because it loses two hydrogens.
E) The alkene is reduced because it gains two hydrogens.

A) Neither, this is not an oxidation-reduction reaction.
B) The alkene is both oxidized and reduced because this is an oxidation-reduction reaction.
C) The alkene is oxidized because it loses two hydrogens.
D) The alkene is reduced because it loses two hydrogens.
E) The alkene is reduced because it gains two hydrogens.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
14
An oxidation-reduction reaction is the transfer of
A) methyl groups.
B) phosphate groups.
C) protons.
D) electrons.
E) oxygen atoms.
A) methyl groups.
B) phosphate groups.
C) protons.
D) electrons.
E) oxygen atoms.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
15
Cellular respiration is an example of a(n) ___________ reaction. I. oxidation-reduction
II) acid-Base
III) combustion
A) I only
B) I and II only
C) III only
D) I and III only
E) I, II and III
II) acid-Base
III) combustion
A) I only
B) I and II only
C) III only
D) I and III only
E) I, II and III

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
16
Ascorbic acid, or vitamin C, is a water soluble vitamin. Which of the following interactions is primarily responsible for ascorbic acid's water solubility? 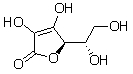
A) dispersion forces
B) dipole-dipole forces
C) hydrogen bonding forces
D) ionic bonding
E) covalent bonding

A) dispersion forces
B) dipole-dipole forces
C) hydrogen bonding forces
D) ionic bonding
E) covalent bonding

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a reason for classifying organic molecules by functional group?
A) The reactivity of a molecule is determined by the functional groups in a molecule.
B) Molecules with the same functional group tend to react similarly.
C) A specific functional group has characteristic reactivity.
D) You can predict the reactivity of a molecule if you know the functional groups that it contains.
E) All of the above
A) The reactivity of a molecule is determined by the functional groups in a molecule.
B) Molecules with the same functional group tend to react similarly.
C) A specific functional group has characteristic reactivity.
D) You can predict the reactivity of a molecule if you know the functional groups that it contains.
E) All of the above

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
18
In an oxidation-reduction reaction, the species that loses electrons is
A) oxygen.
B) oxidized.
C) hydrogen.
D) reduced.
E) carbon.
A) oxygen.
B) oxidized.
C) hydrogen.
D) reduced.
E) carbon.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
19
Which statement best describes why vitamin A is soluble in organic solvents but not in water? 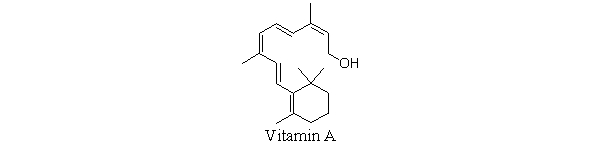
A) Actually, vitamin A is water soluble.
B) The hydroxyl group is hydrophobic in this case.
C) Vitamin A does not contain any hydrophilic groups.
D) The hydrophobic part of the molecule is much larger than the hydrophilic.
E) Vitamin A does not contain any hydrophobic groups.

A) Actually, vitamin A is water soluble.
B) The hydroxyl group is hydrophobic in this case.
C) Vitamin A does not contain any hydrophilic groups.
D) The hydrophobic part of the molecule is much larger than the hydrophilic.
E) Vitamin A does not contain any hydrophobic groups.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
20
In an oxidation-reduction reaction, the species that gains electrons is
A) oxygen.
B) oxidized.
C) hydrogen.
D) reduced.
E) carbon.
A) oxygen.
B) oxidized.
C) hydrogen.
D) reduced.
E) carbon.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
21
The following reaction is the hydrogenation of an unsaturated fatty acid. Which of the following choices best describe molecules A and B? 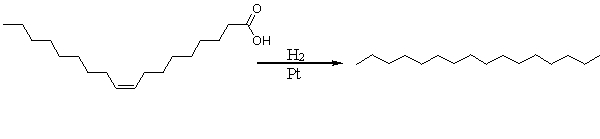
A) Both A and B are fats.
B) Both A and B are oils.
C) A is a fat and B is an oil.
D) A is an oil and B is a fat.
E) A is an oil and B is a trans-fat.

A) Both A and B are fats.
B) Both A and B are oils.
C) A is a fat and B is an oil.
D) A is an oil and B is a fat.
E) A is an oil and B is a trans-fat.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
22
Is the aldehyde oxidized or reduced during the following reaction, and how can you tell? 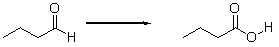
A) Neither. This is not an oxidation-reduction reaction.
B) The aldehyde is neither oxidized nor reduced because the number of hydrogens does not change.
C) The aldehyde is oxidized because it loses two hydrogens.
D) The aldehyde is reduced because it loses two hydrogens.
E) The aldehyde is oxidized because it gains an oxygen.
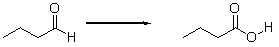
A) Neither. This is not an oxidation-reduction reaction.
B) The aldehyde is neither oxidized nor reduced because the number of hydrogens does not change.
C) The aldehyde is oxidized because it loses two hydrogens.
D) The aldehyde is reduced because it loses two hydrogens.
E) The aldehyde is oxidized because it gains an oxygen.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements describes coenzymes?
A) They are organic molecules derived from vitamins.
B) They are only found in bacterial cells.
C) They are chemically unchanged over the course of a reaction.
D) They are enzymes.
E) They are oxidoreductases.
A) They are organic molecules derived from vitamins.
B) They are only found in bacterial cells.
C) They are chemically unchanged over the course of a reaction.
D) They are enzymes.
E) They are oxidoreductases.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
24
The reaction of fatty acid A is an example of a(n) 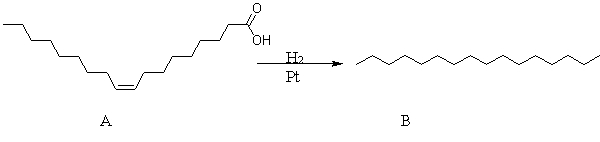
A) neutralization.
B) combustion.
C) oxidation.
D) reduction.
E) condensation.

A) neutralization.
B) combustion.
C) oxidation.
D) reduction.
E) condensation.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
25
Which classification of alcohols cannot be oxidized and why?
A) tertiary, because they don't have a hydrogen on the carbon adjacent to the alcohol
B) tertiary, because the alcohol is already attached to four atoms
C) secondary, because they are too hindered by carbons adjacent to the alcohol
D) primary, because they undergo competing reactions in the presence of an oxidant
E) primary, because the reaction takes too much energy
A) tertiary, because they don't have a hydrogen on the carbon adjacent to the alcohol
B) tertiary, because the alcohol is already attached to four atoms
C) secondary, because they are too hindered by carbons adjacent to the alcohol
D) primary, because they undergo competing reactions in the presence of an oxidant
E) primary, because the reaction takes too much energy

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
26
Which food is NOT high in niacin?
A) oranges
B) anchovies
C) bran
D) peanuts
E) tuna
A) oranges
B) anchovies
C) bran
D) peanuts
E) tuna

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
27
Which vitamin is NAD+/NADH produced from in the body?
A) vitamin C
B) vitamin D
C) niacin
D) vitamin E
E) biotin
A) vitamin C
B) vitamin D
C) niacin
D) vitamin E
E) biotin

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
28
What is the product of the following reaction? 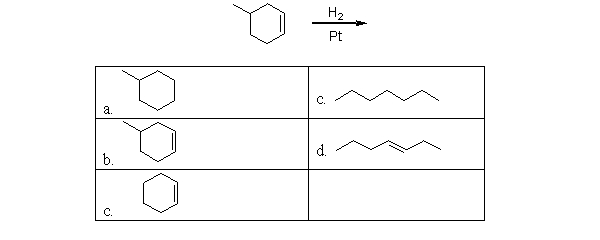
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
29
When ethanol is metabolized, it is
A) synthesized.
B) oxidized.
C) anabolized.
D) substituted.
E) reduced.
A) synthesized.
B) oxidized.
C) anabolized.
D) substituted.
E) reduced.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
30
When unsaturated fatty acids are hydrogenated, an isomerization reaction sometimes occurs producing
A) saturated fatty acids.
B) unsaturated fatty acid.
C) cis-fats.
D) trans-fats.
E) carboxylate ions.
A) saturated fatty acids.
B) unsaturated fatty acid.
C) cis-fats.
D) trans-fats.
E) carboxylate ions.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
31
Foods that contain the product of the reaction shown below are labeled as 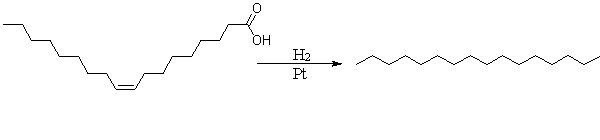
A) hydrogenated or partially hydrogenated.
B) fatty.
C) oily.
D) fat free.
E) diet.

A) hydrogenated or partially hydrogenated.
B) fatty.
C) oily.
D) fat free.
E) diet.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
32
Which functional group can be oxidized but not reduced?
A) an alcohol
B) an alkane
C) an alkyne
D) an alkene
E) a carboxylic acid
A) an alcohol
B) an alkane
C) an alkyne
D) an alkene
E) a carboxylic acid

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
33
What is the purpose of NAD+ in the body?
A) It is an oxidizing agent.
B) It is a reducing agent.
C) It donates oxygen.
D) It donates hydrogen.
E) It accepts oxygen.
A) It is an oxidizing agent.
B) It is a reducing agent.
C) It donates oxygen.
D) It donates hydrogen.
E) It accepts oxygen.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
34
Sorbitol is a sweetener often used in chewing gum and diet drinks. It is obtained by the following reaction. Which of the following functional group transformations occur during this reaction? 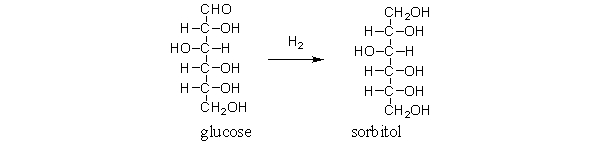
A) The aldehyde becomes an alcohol.
B) An alcohol becomes an aldehyde.
C) An alcohol disappears.
D) A new aldehyde appears.
E) All of the above

A) The aldehyde becomes an alcohol.
B) An alcohol becomes an aldehyde.
C) An alcohol disappears.
D) A new aldehyde appears.
E) All of the above

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
35
Which statement best describes the following reaction? 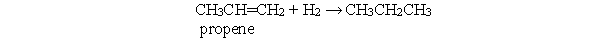
A) In this reaction, both propene and hydrogen are reduced.
B) In this reaction, both propene and hydrogen are oxidized.
C) In this reaction, propene is reduced and hydrogen is oxidized.
D) In this reaction, propene is oxidized and hydrogen is reduced.
E) This is not an oxidation-reduction reaction.
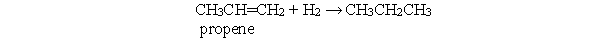
A) In this reaction, both propene and hydrogen are reduced.
B) In this reaction, both propene and hydrogen are oxidized.
C) In this reaction, propene is reduced and hydrogen is oxidized.
D) In this reaction, propene is oxidized and hydrogen is reduced.
E) This is not an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
36
What is the product when this compound undergoes oxidation? 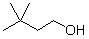
A)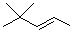
B)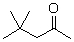
C)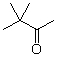
D)
E)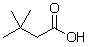

A)

B)
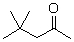
C)

D)

E)


Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
37
Sorbitol is a sweetener often used in chewing gum and diet drinks. It is obtained by the following reaction. Is glucose oxidized or reduced during the reaction? 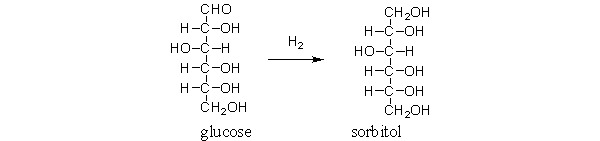
A) It is oxidized.
B) It is reduced.
C) It is neither oxidized nor reduced.
D) It is both oxidized and reduced.
E) Many different reactions occur.

A) It is oxidized.
B) It is reduced.
C) It is neither oxidized nor reduced.
D) It is both oxidized and reduced.
E) Many different reactions occur.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
38
Is the ketone oxidized or reduced during this reaction and how can you tell? 
A) Neither. This is not an oxidation-reduction reaction.
B) The ketone is both oxidized and reduced because this is an oxidation-reduction reaction.
C) The ketone is oxidized because it loses two hydrogens.
D) The ketone is reduced because it loses two hydrogens.
E) The ketone is reduced because it gains two hydrogens.

A) Neither. This is not an oxidation-reduction reaction.
B) The ketone is both oxidized and reduced because this is an oxidation-reduction reaction.
C) The ketone is oxidized because it loses two hydrogens.
D) The ketone is reduced because it loses two hydrogens.
E) The ketone is reduced because it gains two hydrogens.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
39
Carbohydrates are molecules that are composed of many oxygen-containing functional groups. Carbohydrates, like other oxygen-containing molecules, can be oxidized in the laboratory. Which of the following choices illustrates the oxidation of mannose, a simple carbohydrate? 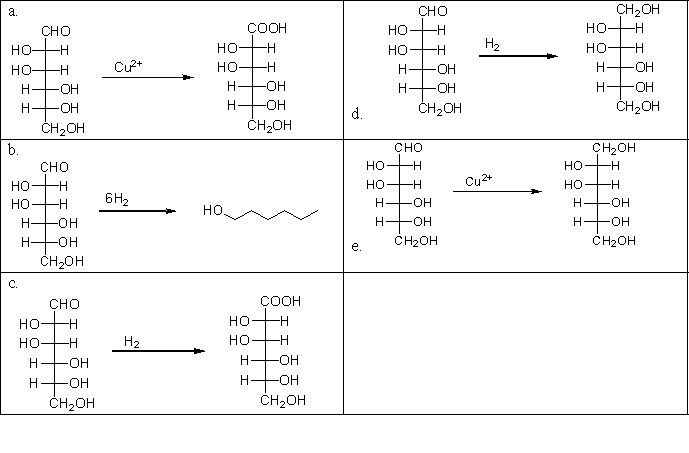
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
40
Which classification of alcohols can undergo oxidation to yield a ketone?
A) Both primary and secondary alcohols yield ketones when oxidized.
B) Both secondary and tertiary alcohols yield ketones when oxidized.
C) Only primary alcohols yield ketones when oxidized.
D) Only secondary alcohols yield ketones when oxidized.
E) Only tertiary alcohols yield ketones when oxidized.
A) Both primary and secondary alcohols yield ketones when oxidized.
B) Both secondary and tertiary alcohols yield ketones when oxidized.
C) Only primary alcohols yield ketones when oxidized.
D) Only secondary alcohols yield ketones when oxidized.
E) Only tertiary alcohols yield ketones when oxidized.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
41
The following reaction is one step in the key metabolic pathway called the TCA (or Krebs) cycle. This reaction is missing a reactant (A) which reacts to form a product (B). What is the identity of A and B? 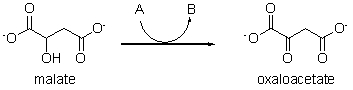
A) A is NAD+ and B is NADH + H+.
B) A is FAD and B is FADH2.
C) A is NADH + H+ and B is NAD+.
D) A is FADH2 and B is FAD.
E) Actually, there is no A, but B is H2.

A) A is NAD+ and B is NADH + H+.
B) A is FAD and B is FADH2.
C) A is NADH + H+ and B is NAD+.
D) A is FADH2 and B is FAD.
E) Actually, there is no A, but B is H2.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following statements describe esters? I. They are fruit flavorings.
II) They are sometimes a component of perfumes.
III) Soap can be made from an ester.
A) All of these statements describe esters.
B) Only I describes esters.
C) I and II describe esters.
D) I and III describe esters.
E) II and III describe esters.
II) They are sometimes a component of perfumes.
III) Soap can be made from an ester.
A) All of these statements describe esters.
B) Only I describes esters.
C) I and II describe esters.
D) I and III describe esters.
E) II and III describe esters.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
43
The following reaction occurs in the body. Which of the following reactants is most probably required for this reaction to occur? 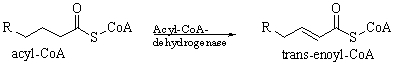
A) O2
B) H2
C) FAD
D) NAD
E) FADH2

A) O2
B) H2
C) FAD
D) NAD
E) FADH2

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is a free radical?
A) ".OH"
B) "HCOO-"
C) "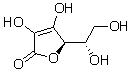
Vitamin C"
D) Mg2+"
E) O2"
A) ".OH"
B) "HCOO-"
C) "

Vitamin C"
D) Mg2+"
E) O2"

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following partial structures is an acetyl group? 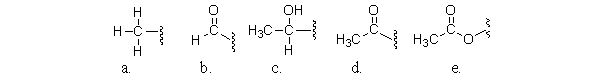
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
46
Which statement best describes what occurs over the course of the following reaction? 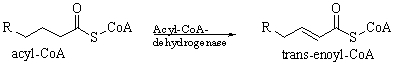
A) Two hydrogens are added to acyl-CoA, forming a double bond.
B) Two hydrogens are added to acyl-CoA, breaking a double bond.
C) Two protons are added to acyl-CoA, forming a double bond.
D) Two hydrogens are removed from acyl-CoA, forming a double bond.
E) Two protons are removed from acyl-CoA, forming a double bond.

A) Two hydrogens are added to acyl-CoA, forming a double bond.
B) Two hydrogens are added to acyl-CoA, breaking a double bond.
C) Two protons are added to acyl-CoA, forming a double bond.
D) Two hydrogens are removed from acyl-CoA, forming a double bond.
E) Two protons are removed from acyl-CoA, forming a double bond.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
47
What is the purpose of flavin adenine dinucleotide (FAD) in the body?
A) It is an oxidizing agent.
B) It is a reducing agent.
C) It donates oxygen.
D) It donates hydrogen.
E) It accepts oxygen.
A) It is an oxidizing agent.
B) It is a reducing agent.
C) It donates oxygen.
D) It donates hydrogen.
E) It accepts oxygen.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
48
What is the name of the functional group produced by the following reaction? 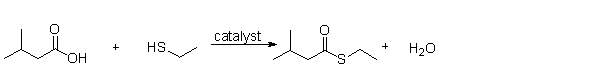
A) a thioester
B) a sulfur
C) a sulfhydride
D) a thiol
E) a hydrogen sulfide

A) a thioester
B) a sulfur
C) a sulfhydride
D) a thiol
E) a hydrogen sulfide

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following species is NOT a free radical?
A) "HO."
B) "HOO."
C) "CH4"
D) "O2.-"
E) ".CH3"
A) "HO."
B) "HOO."
C) "CH4"
D) "O2.-"
E) ".CH3"

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
50
What is the role of antioxidants in the body?
A) to reduce free radicals before they can damage cells
B) to prevent metabolism from occurring
C) to make free radicals
D) to react with coenzymes
E) All of the above
A) to reduce free radicals before they can damage cells
B) to prevent metabolism from occurring
C) to make free radicals
D) to react with coenzymes
E) All of the above

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following functional groups is produced when a carboxylic acid and an alcohol react in the presence of a catalyst?
A) an aldehyde
B) an ether
C) a ketone
D) an ester
E) a salt
A) an aldehyde
B) an ether
C) a ketone
D) an ester
E) a salt

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
52
An antioxidant is a substance that prevents ____________ of other cellular substances.
A) all reduction
B) harmful reduction
C) all oxidation
D) harmful oxidation
E) both oxidation and reduction
A) all reduction
B) harmful reduction
C) all oxidation
D) harmful oxidation
E) both oxidation and reduction

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
53
FAD and NAD+ are examples of
A) enzymes.
B) coenzymes.
C) vitamins.
D) substrates.
E) inhibitors.
A) enzymes.
B) coenzymes.
C) vitamins.
D) substrates.
E) inhibitors.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
54
The following reaction is an example of a(n) 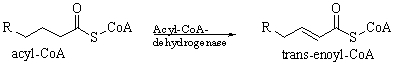
A) neutralization.
B) combustion.
C) oxidation.
D) reduction.
E) condensation.

A) neutralization.
B) combustion.
C) oxidation.
D) reduction.
E) condensation.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
55
The following reaction shows the oxidation-reduction reaction of FAD/FADH2. Which choice correctly identifies each molecule? 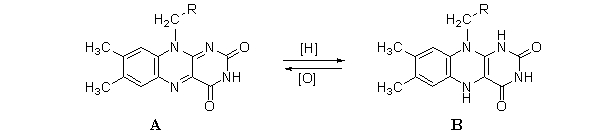
A)a
B)b
C)c
D)d
E)e


A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
56
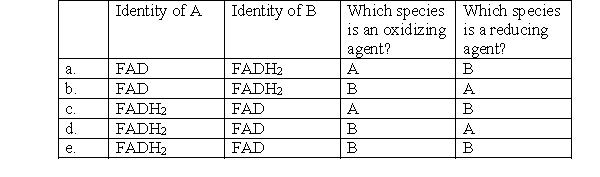
What is the missing reactant for the following reaction? 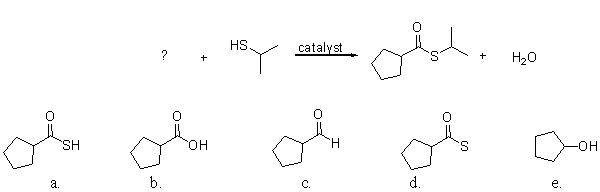
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
57
The reaction of a carboxylic acid and an alcohol in the presence of a catalyst produces an ester and a molecule of water. Which of the labeled atoms below are involved in making the molecule of water? 
A) a and d
B) b and d
C) b, c, and d
D) a, c, and d
E) c, d, and e
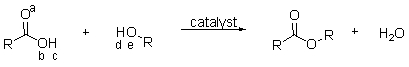
A) a and d
B) b and d
C) b, c, and d
D) a, c, and d
E) c, d, and e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
58
Which statement describes a difference between FAD/FADH2 ?and NAD+/NADH?
A) FAD/FADH2 is an enzyme and NAD+/NADH is not.
B) FAD/FADH2 is coenzyme and NAD+/NADH is not.
C) FAD/FADH2 is found in cells of the body and NAD+/NADH is not.
D) FAD/FADH2 is involved in reactions of alkenes and NAD+/NADH is involved in reactions of C-O bonds.
E) FAD/FADH2 and NAD+/NADH are identical.
A) FAD/FADH2 is an enzyme and NAD+/NADH is not.
B) FAD/FADH2 is coenzyme and NAD+/NADH is not.
C) FAD/FADH2 is found in cells of the body and NAD+/NADH is not.
D) FAD/FADH2 is involved in reactions of alkenes and NAD+/NADH is involved in reactions of C-O bonds.
E) FAD/FADH2 and NAD+/NADH are identical.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
59
The first two steps of ethanol metabolism are shown below. Which molecule is reduced over the course of these reactions? 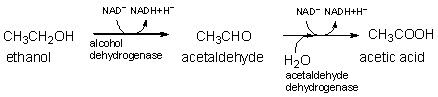
A) ethanol and acetaldehyde
B) alcohol dehydrogenase and acetaldehyde dehydrogenase
C) ethanol and alcohol dehydrogenase
D) acetic acid
E) NAD+
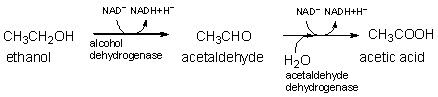
A) ethanol and acetaldehyde
B) alcohol dehydrogenase and acetaldehyde dehydrogenase
C) ethanol and alcohol dehydrogenase
D) acetic acid
E) NAD+

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following partial structures is an acyl group? 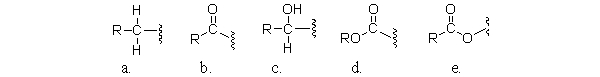
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
61
An ester, thioester or amide can all undergo hydrolysis. Which of the following characteristics do these three reactions have in common?
A) All produce water.
B) All produce a carboxylic acid.
C) All require a strong acid to occur.
D) All are very unfavorable.
E) All are very fast reactions.
A) All produce water.
B) All produce a carboxylic acid.
C) All require a strong acid to occur.
D) All are very unfavorable.
E) All are very fast reactions.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
62
What are the products of the following reaction? 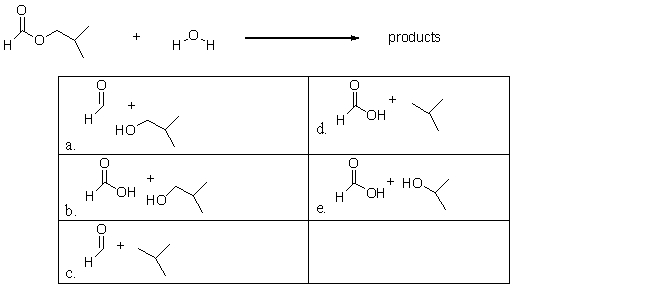
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following reactions is used in making soap? 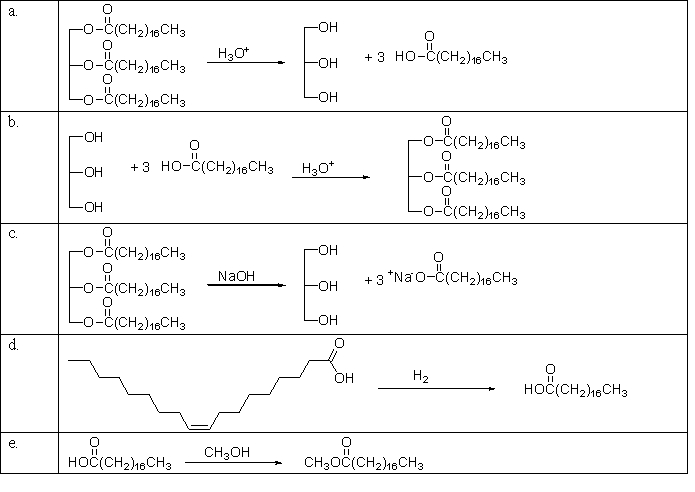
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
64
During hydrolysis reactions, like all reactions, some bonds are broken and new ones are formed. Which bonds are broken and which are formed during the following hydrolysis reaction? 
A) Broken: a-b only Formed: e-a only
B) Broken: b-c only Formed: b-d only
C) Broken: b-c and d-e Formed: b-d and c-e
D) Broken: a-b and b-c Formed: a-e and c-e
E) Broken: a-b and e-d Formed: a-e and b-d

A) Broken: a-b only Formed: e-a only
B) Broken: b-c only Formed: b-d only
C) Broken: b-c and d-e Formed: b-d and c-e
D) Broken: a-b and b-c Formed: a-e and c-e
E) Broken: a-b and e-d Formed: a-e and b-d

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
65
What is the product of this esterification reaction? 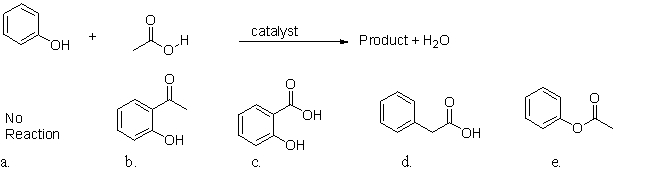
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following reactions is the saponification of a fat? 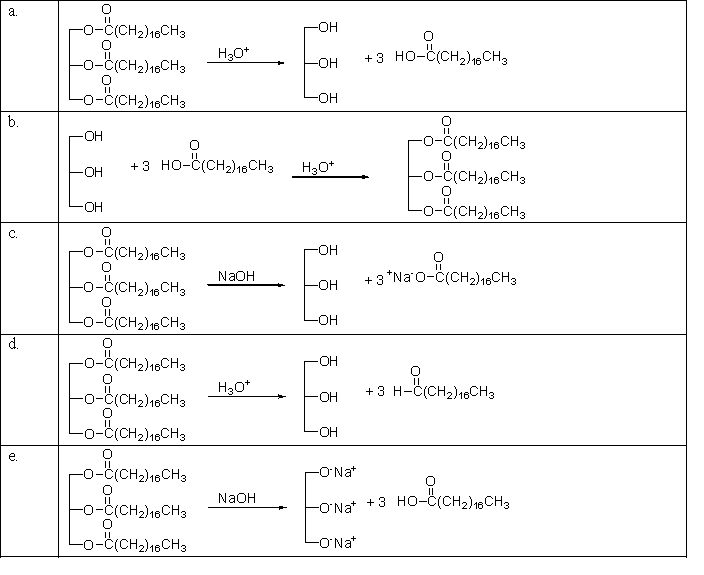
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
67
After an amide is hydrolyzed, it undergoes further reaction. Which of the following occurs after an amide is hydrolyzed?
A) hydration
B) dehydration
C) an acid-base reaction
D) another hydrolysis
E) condensation
A) hydration
B) dehydration
C) an acid-base reaction
D) another hydrolysis
E) condensation

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
68
Which ester has a line through the bond(s) that is broken during hydrolysis? 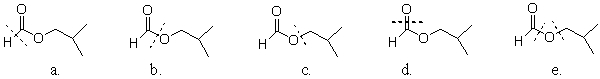
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
69
Which statement best describes the distinction between a hydration and a dehydration reaction?
A) Hydration requires a carbonyl group and dehydration does not.
B) Hydration is the addition of water and dehydration is the loss of water.
C) Hydration results in breaking bonds and dehydration creates bonds.
D) Hydration is an oxidation and dehydration is a reduction.
E) Hydration and dehydration actually describe the same process.
A) Hydration requires a carbonyl group and dehydration does not.
B) Hydration is the addition of water and dehydration is the loss of water.
C) Hydration results in breaking bonds and dehydration creates bonds.
D) Hydration is an oxidation and dehydration is a reduction.
E) Hydration and dehydration actually describe the same process.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
70
Which amide has a line through the bond that is broken during hydrolysis? 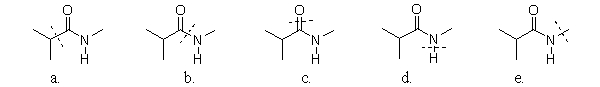
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
71
Hydrolysis is an example of an acyl group transfer reaction. To what molecule is an acyl group transferred during this type of reaction?
A) an amine
B) -OH
C) a carboxylic acid
D) a thiol
E) an ether
A) an amine
B) -OH
C) a carboxylic acid
D) a thiol
E) an ether

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
72
What type of reaction is this? 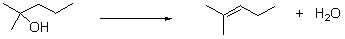
A) oxidation
B) reduction
C) combustion
D) hydration
E) dehydration

A) oxidation
B) reduction
C) combustion
D) hydration
E) dehydration

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
73
What is the product of the following reaction? 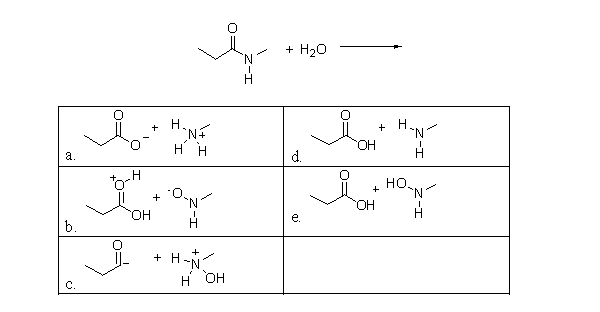
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
74
What is the product of the following amidation reaction? 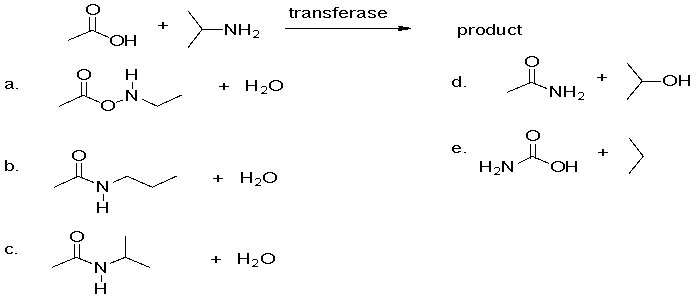
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
75
An amide is hydrolyzed as is shown in the following reaction. After it is hydrolyzed, it undergoes another reaction. Which of the following choices represent the products of this further reaction? 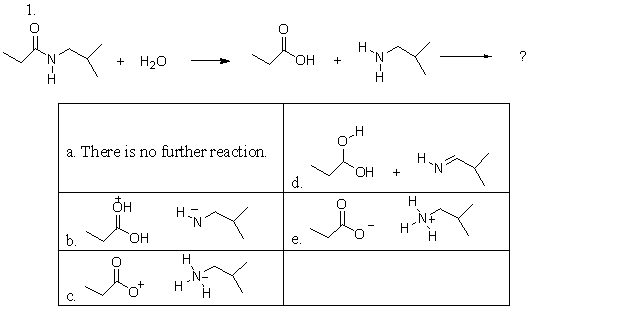
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is the missing reactant in the reaction below, the hydrolysis of a thioester? 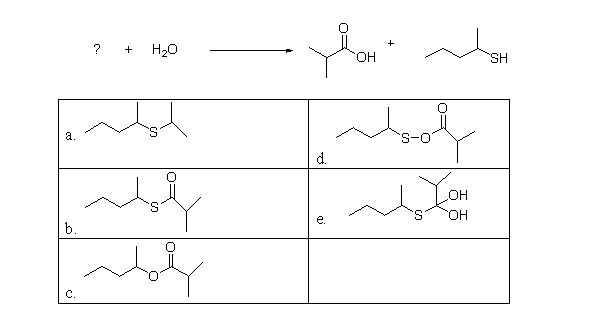
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
77
Which statement best describes a similarity between a hydration and a dehydration reaction?
A) They both involve hydrogen.
B) They both involve addition to a double bond.
C) They both result in splitting a molecule.
D) The both result in forming one molecule from two.
E) They both involve water.
A) They both involve hydrogen.
B) They both involve addition to a double bond.
C) They both result in splitting a molecule.
D) The both result in forming one molecule from two.
E) They both involve water.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
78
What is the product of the following hydrolysis reaction between an ester and water? 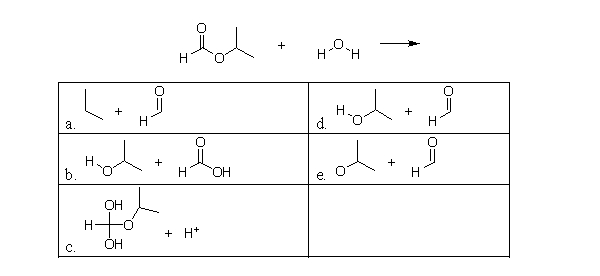
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
79
What type of reaction is this? 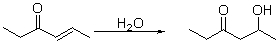
A) oxidation
B) reduction
C) combustion
D) hydration
E) dehydration
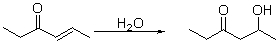
A) oxidation
B) reduction
C) combustion
D) hydration
E) dehydration

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
80
What is the product of the following reaction? 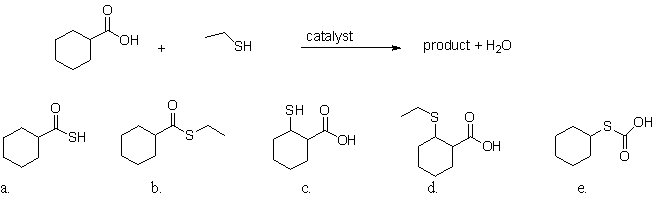
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck


