Deck 1: Measuring Matter and Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 1: Measuring Matter and Energy
1
This state of matter has the highest kinetic energy.
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas
gas
2
The illustration below shows two metal blocks, one hot and one cold, placed together so their sides are touching. What do you expect to happen to the temperature of the blocks as time passes? 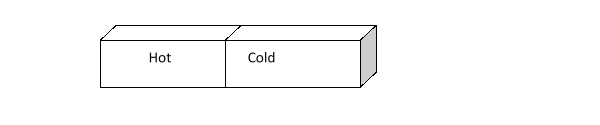
A) Nothing
B) The temperature of the hot block will decrease and the temperature of the cold block will increase a little bit, but the hot block will always stay a bit warmer than the cold one.
C) The temperature of the cold block will decrease, and the temperature of the hot block will increase.
D) The hot block will cool down, but the temperature of the cold block will not change.
E) The temperature of the cold block will increase, and the temperature of the hot block will decrease until the temperature of the two blocks is the same.

A) Nothing
B) The temperature of the hot block will decrease and the temperature of the cold block will increase a little bit, but the hot block will always stay a bit warmer than the cold one.
C) The temperature of the cold block will decrease, and the temperature of the hot block will increase.
D) The hot block will cool down, but the temperature of the cold block will not change.
E) The temperature of the cold block will increase, and the temperature of the hot block will decrease until the temperature of the two blocks is the same.
The temperature of the cold block will increase, and the temperature of the hot block will decrease until the temperature of the two blocks is the same.
3
Which of the following is NOT an example of a physical change?
A) freezing water to make ice
B) ice cream melting in a bowl
C) boiling water
D) water condensing on the surface of a glass of ice tea
E) onions browning as they are cooked
A) freezing water to make ice
B) ice cream melting in a bowl
C) boiling water
D) water condensing on the surface of a glass of ice tea
E) onions browning as they are cooked
onions browning as they are cooked
4
A person with a T-score of less than 2.5 has a bone mineral density that ______ normal and is said to have ______.
A) is; normal bone density
B) is less than; normal bone density
C) is less than; osteoporosis
D) is greater than; osteopenia
E) is; osteopenia
A) is; normal bone density
B) is less than; normal bone density
C) is less than; osteoporosis
D) is greater than; osteopenia
E) is; osteopenia

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
Which state of matter has a volume that is constant or fixed?
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following describes the potential energy of an object or set of objects?
A) water flowing down hill
B) water is a reservoir
C) a person running the 50-yard dash
D) a car speeding up a hill
E) a student pushing open a door
A) water flowing down hill
B) water is a reservoir
C) a person running the 50-yard dash
D) a car speeding up a hill
E) a student pushing open a door

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
The illustration below shows two metal blocks, one hot and one cold, placed together so their sides are touching. How does atomic motion change as time passes 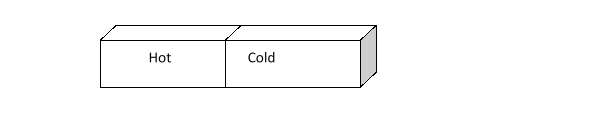
A) Atomic motion does not change as time passes.
B) Atomic motion does change, but it is not predictable how it will change.
C) Atoms in the hot block slow down, and atoms in the cold block speed up.
D) Atoms in the cold block slow down, and atoms in the hot block speed up.
E) Atoms in both the cold and hot block speed up.

A) Atomic motion does not change as time passes.
B) Atomic motion does change, but it is not predictable how it will change.
C) Atoms in the hot block slow down, and atoms in the cold block speed up.
D) Atoms in the cold block slow down, and atoms in the hot block speed up.
E) Atoms in both the cold and hot block speed up.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
How does the kinetic energy of the hot and cold bricks below change as time passes? 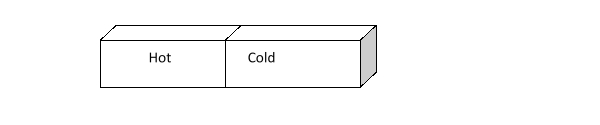
A) The kinetic energy of the bricks does not change as time passes.
B) Kinetic energy increases in both blocks.
C) Kinetic energy in the hot block decreases and kinetic energy in the cold block increases.
D) Kinetic energy in the hot block increases and kinetic energy in the cold block decreases.
E) Kinetic energy decreases in both blocks.

A) The kinetic energy of the bricks does not change as time passes.
B) Kinetic energy increases in both blocks.
C) Kinetic energy in the hot block decreases and kinetic energy in the cold block increases.
D) Kinetic energy in the hot block increases and kinetic energy in the cold block decreases.
E) Kinetic energy decreases in both blocks.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
The speed of molecules and atoms in this state of matter is the slowest.
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
Identify whether the following represent the microscopic, macroscopic, or atomic scale. i. Hemoglobin ii. Person iii. Red blood cell
A)
B)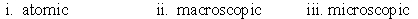
C)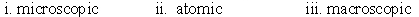
D)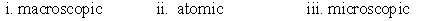
E)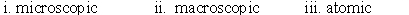
A)
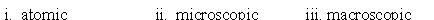
B)
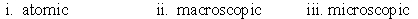
C)
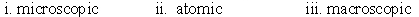
D)
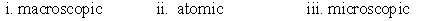
E)
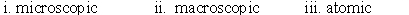

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following describes the kinetic energy of an object or set of objects?
A) water flowing down hill
B) water in a reservoir
C) the forces between two molecules
D) the chemical bonds in a peanut butter and jelly sandwich
E) a book on top of a shelf
A) water flowing down hill
B) water in a reservoir
C) the forces between two molecules
D) the chemical bonds in a peanut butter and jelly sandwich
E) a book on top of a shelf

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
A molecule of hemoglobin is described as being on the _____ scale.
A) macroscopic
B) microscopic
C) atomic
D) both macro- and microscopic
E) both microscopic and atomic
A) macroscopic
B) microscopic
C) atomic
D) both macro- and microscopic
E) both microscopic and atomic

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
Below are five descriptions of the kinetic and potential energy of objects. Which is a description of potential energy?
I. water moving a waterwheel
II. a skateboarder at the top of a halfpipe
III. the blades of a fan turning
IV. hot water molecules moving rapidly in a cup of tea
V. a parachutist ready to jump out of a plane
A) I only
B) II and V
C) II, III, and V
D) I, III, and IV
E) All of the above
I. water moving a waterwheel
II. a skateboarder at the top of a halfpipe
III. the blades of a fan turning
IV. hot water molecules moving rapidly in a cup of tea
V. a parachutist ready to jump out of a plane
A) I only
B) II and V
C) II, III, and V
D) I, III, and IV
E) All of the above

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Heat is _________ energy, while temperature is a _________________.
A) potential; measure of potential energy
B) kinetic; measure of kinetic energy
C) potential; measure of kinetic energy
D) kinetic; measure of potential energy
E) Actually, both heat and temperature are forms of kinetic energy.
A) potential; measure of potential energy
B) kinetic; measure of kinetic energy
C) potential; measure of kinetic energy
D) kinetic; measure of potential energy
E) Actually, both heat and temperature are forms of kinetic energy.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
Label each box with the appropriate state of matter. 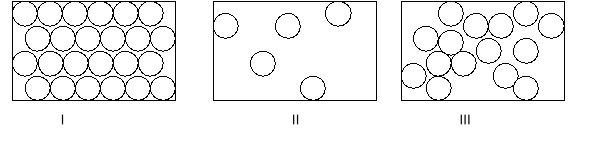
A) I: gas II: liquid III: solid
B) I: liquid II: solid III: gas
C) I: solid II: liquid III: gas
D) I: gas II: solid III: liquid
E) I: solid II: gas III: liquid

A) I: gas II: liquid III: solid
B) I: liquid II: solid III: gas
C) I: solid II: liquid III: gas
D) I: gas II: solid III: liquid
E) I: solid II: gas III: liquid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
This state of matter changes shape depending upon the shape of its container.
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
Chemistry attempts to explain the behavior of matter on the _____ so that we can better understand the properties of matter that we observe on the _____.
A) microscopic scale; macroscopic scale
B) microscopic scale; atomic scale
C) atomic scale; microscopic scale
D) macroscopic scale; atomic scale
E) atomic scale; macroscopic scale
A) microscopic scale; macroscopic scale
B) microscopic scale; atomic scale
C) atomic scale; microscopic scale
D) macroscopic scale; atomic scale
E) atomic scale; macroscopic scale

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Below are five descriptions of the kinetic and potential energy of objects. Which is a description of kinetic energy?
I. water moving a waterwheel
II. a skateboarder at the top of a halfpipe
III. the blades of a fan turning
IV. hot water molecules moving rapidly in a cup of tea
V. a parachutist ready to jump out of a plane
A) I only
B) II and V
C) II, III, and V
D) I, III, and IV
E) All of the above
I. water moving a waterwheel
II. a skateboarder at the top of a halfpipe
III. the blades of a fan turning
IV. hot water molecules moving rapidly in a cup of tea
V. a parachutist ready to jump out of a plane
A) I only
B) II and V
C) II, III, and V
D) I, III, and IV
E) All of the above

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
Atoms and molecules in this state of matter are the most highly ordered.
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas
A) solid
B) liquid
C) gas
D) solid and liquid
E) liquid and gas

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
How are physical changes different than chemical changes?
A) They aren't different at all.
B) Chemical changes are much more common than physical changes.
C) Chemical changes are much faster than physical changes.
D) Chemical changes involve making and breaking bonds and physical changes do not.
E) Chemical changes involve changes of state and physical changes do not.
A) They aren't different at all.
B) Chemical changes are much more common than physical changes.
C) Chemical changes are much faster than physical changes.
D) Chemical changes involve making and breaking bonds and physical changes do not.
E) Chemical changes involve changes of state and physical changes do not.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
What is being measured by the ruler (ruler A)? 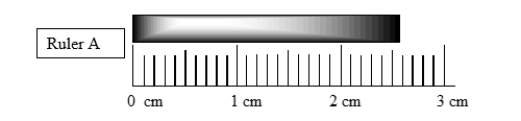
A) volume
B) weight
C) grams
D) length
E) temperature

A) volume
B) weight
C) grams
D) length
E) temperature

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following units of energy is equal to one thousand calories?
A) 1 Calorie only
B) 1 kcal only
C) 1 joule only
D) 1 kcal and 1 Calorie
E) 1 kcal and 1 kJ
A) 1 Calorie only
B) 1 kcal only
C) 1 joule only
D) 1 kcal and 1 Calorie
E) 1 kcal and 1 kJ

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
The cubic centimeter (cm3 or cc) is the same volume as a
A) centimeter.
B) milliliter.
C) centiliter.
D) deciliter.
E) liter.
A) centimeter.
B) milliliter.
C) centiliter.
D) deciliter.
E) liter.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is most likely to be 1.1 m tall?
A) a giraffe
B) a 5-year-old girl
C) a man
D) an infant
E) All four are equally likely to be 1.1 m tall.
A) a giraffe
B) a 5-year-old girl
C) a man
D) an infant
E) All four are equally likely to be 1.1 m tall.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
A juice box is 10.0 cm 5.5 cm 4.0 cm. What is the maximum amount of juice that the box can contain?
A) 220 cm3
B) 110 cm3
C) 220 cm2
D) 110 cm2
E) 220 cm
A) 220 cm3
B) 110 cm3
C) 220 cm2
D) 110 cm2
E) 220 cm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
How long is the bar above the ruler? 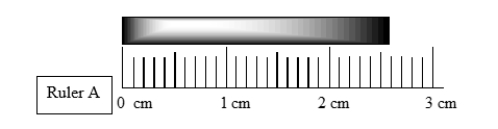
A) 2.5 cm
B) 2.6 cm
C) 2.59 cm
D) 3 cm
E) 2 cm

A) 2.5 cm
B) 2.6 cm
C) 2.59 cm
D) 3 cm
E) 2 cm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following measurements includes a base unit?
A) 298 mg
B) 2.981 g
C) 5 103 kg
D) 3.6 mL
E) 168 mm
A) 298 mg
B) 2.981 g
C) 5 103 kg
D) 3.6 mL
E) 168 mm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is most likely to weigh 90 kg?
A) a computer
B) a zebra
C) a man
D) a baby girl
E) All four are equally likely to weigh 90 kg.
A) a computer
B) a zebra
C) a man
D) a baby girl
E) All four are equally likely to weigh 90 kg.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is likely to be shorter than 1 m?
A) the length of a car
B) the height of an average adult
C) the width of a computer screen
D) the height of a one-story building
E) the length of an adult giraffe's neck
A) the length of a car
B) the height of an average adult
C) the width of a computer screen
D) the height of a one-story building
E) the length of an adult giraffe's neck

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
An aluminum ball is dropped into the graduated cylinder and the water level increases. If the ball has a volume of 6.8 mL, what is the new volume reading in the graduated cylinder?
A) 6.8 mL
B) 83 mL
C) 90.7 mL
D) 96.8 mL
E) It is not possible to predict the volume without the density of aluminum.
A) 6.8 mL
B) 83 mL
C) 90.7 mL
D) 96.8 mL
E) It is not possible to predict the volume without the density of aluminum.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
There are five different objects with the diameters shown below. Which of these objects cannot be seen with the naked eye?
A) 1.0 nm
B) 1.0 mm
C) 1.0 μm
D) 1.0 dm
E) 1.0 cm
A) 1.0 nm
B) 1.0 mm
C) 1.0 μm
D) 1.0 dm
E) 1.0 cm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following measurements represents the least mass?
A) 0.1 mg
B) 1000 μg
C) 0.001 g
D) 1 cg
E) 0.010 kg
A) 0.1 mg
B) 1000 μg
C) 0.001 g
D) 1 cg
E) 0.010 kg

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following units for measuring energy is typically used in nutritional applications?
A) calorie
B) Calorie
C) Joule
D) joule
E) All of the above
A) calorie
B) Calorie
C) Joule
D) joule
E) All of the above

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements best describes the unit "milliliter"?
A) It is a prefix followed by a base unit.
B) It is a base unit.
C) It is a prefix followed by a derived unit.
D) It is a derived unit.
E) It is a base unit followed by a suffix.
A) It is a prefix followed by a base unit.
B) It is a base unit.
C) It is a prefix followed by a derived unit.
D) It is a derived unit.
E) It is a base unit followed by a suffix.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following measurements is larger than 1.0 meters?
A) 10 cm
B) 0.0001 km
C) 0.01 km
D) 100 mm
E) 1000 μm
A) 10 cm
B) 0.0001 km
C) 0.01 km
D) 100 mm
E) 1000 μm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following equalities is NOT correct?
A) 1 cm = 10-2 m
B) 103 g = 1 kg
C) 10-3 mL = 1 L
D) 109 nm = 1 m
E) 1 L = 10 dL
A) 1 cm = 10-2 m
B) 103 g = 1 kg
C) 10-3 mL = 1 L
D) 109 nm = 1 m
E) 1 L = 10 dL

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
A graduated cylinder contains water with some food coloring in it. What is being measured?
A) volume
B) weight
C) distance
D) length
E) temperature
A) volume
B) weight
C) distance
D) length
E) temperature

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
Every measurement consists of
A) a number followed by a unit.
B) only whole numbers.
C) a fraction.
D) a number followed by a description of the device used to take the measurement.
E) There are no characteristics that all measurements share.
A) a number followed by a unit.
B) only whole numbers.
C) a fraction.
D) a number followed by a description of the device used to take the measurement.
E) There are no characteristics that all measurements share.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following equalities is correct?
A) 1 mL = 1 cm3
B) 1 L = 1 cm3
C) 1 mL = 1 cm2
D) 1 L = 1 cm2
E) 1 mL = 1 cm
A) 1 mL = 1 cm3
B) 1 L = 1 cm3
C) 1 mL = 1 cm2
D) 1 L = 1 cm2
E) 1 mL = 1 cm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
How many nanometers are in a meter?
A) 1 x 10-9
B) 1 x 109
C) 1 x 10-12
D) 1 x 103
E) 1 x 10-6
A) 1 x 10-9
B) 1 x 109
C) 1 x 10-12
D) 1 x 103
E) 1 x 10-6

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following measurements has three significant figures?
A) 0.0058 m
B) 580.0 m
C) 5800 m
D) 0.058 m
E) 0.0580 m
A) 0.0058 m
B) 580.0 m
C) 5800 m
D) 0.058 m
E) 0.0580 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
To make this table, four different people took three measurements each of the distance between the chemistry building and the cafeteria on campus. Each person used a different measuring device and therefore arrived at a different set of measurements. The true distance is 152 meters. Which person is the least precise and the least accurate? 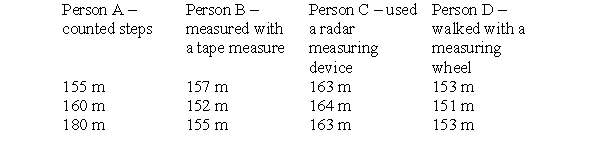
A) person A
B) person B
C) person C
D) person D
E) All are equally inaccurate and imprecise.

A) person A
B) person B
C) person C
D) person D
E) All are equally inaccurate and imprecise.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
The number of significant digits in a measurement is
A) all of the digits that are known exactly.
B) all of the digits that are known exactly plus one uncertain digit.
C) a way to communicate the precision of a measurement.
D) Both a and c above
E) Both b and c above
A) all of the digits that are known exactly.
B) all of the digits that are known exactly plus one uncertain digit.
C) a way to communicate the precision of a measurement.
D) Both a and c above
E) Both b and c above

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is a measured number?
A) the number of eggs in a dozen
B) the number of people in this room
C) the number of milligrams in a gram
D) the number of years in a century
E) the number of grams in one ounce
A) the number of eggs in a dozen
B) the number of people in this room
C) the number of milligrams in a gram
D) the number of years in a century
E) the number of grams in one ounce

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
Using significant figures, what is the product of 0.021 0.118 1020?
A) 2.52756
B) 2.528
C) 2.53
D) 2.5
E) 3
A) 2.52756
B) 2.528
C) 2.53
D) 2.5
E) 3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
The following illustrates the digital readout of two different balances. Which of the following balances is more precise? Which is more accurate? 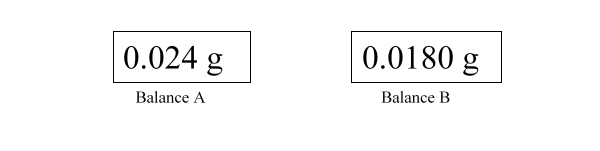
A) Balance A is more precise and more accurate than balance B
B) Balance B is more accurate than balance A, but less precise.
C) Balance A is more accurate than balance B, but less precise.
D) Balance B is more precise and more accurate than balance A
E) Balance B is more precise than balance A, but it is not possible to compare accuracy based on the information given.

A) Balance A is more precise and more accurate than balance B
B) Balance B is more accurate than balance A, but less precise.
C) Balance A is more accurate than balance B, but less precise.
D) Balance B is more precise and more accurate than balance A
E) Balance B is more precise than balance A, but it is not possible to compare accuracy based on the information given.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
You are asked to administer 3.50 mL of a liquid medication. Which measuring device would be the best choice for measuring 3.50 mL?
A) A medicine cup
B) A syringe
C) A medicine cup and a syringe are equally good choices.
D) It would depend on the composition of the medication.
E) It is not possible to determine the answer with the given information.
A) A medicine cup
B) A syringe
C) A medicine cup and a syringe are equally good choices.
D) It would depend on the composition of the medication.
E) It is not possible to determine the answer with the given information.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
To make this table, four different people took three measurements each of the distance between the chemistry building and the cafeteria on campus. Each person used a different measuring device and therefore arrived at a different set of measurements. The true distance is 152 meters. Which person is precise, but not very accurate? 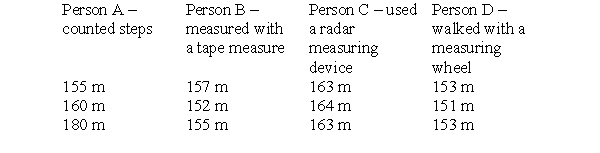
A) person A
B) person B
C) person C
D) person D
E) All are equally precise.

A) person A
B) person B
C) person C
D) person D
E) All are equally precise.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
A patient is given 5.00 mL of a medication that contains 0.0012 g of active ingredient per mL. To determine the amount of active ingredient administered, the product of the two numbers is calculated (5.00 mL 0.0012 g/mL). Using significant figures, what is this product?
A) 0.006 g
B) 0.0060 g
C) 0.00600 g
D) 6.00 g
E) 0 g
A) 0.006 g
B) 0.0060 g
C) 0.00600 g
D) 6.00 g
E) 0 g

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
A patient's fluid intake is monitored over a six-hour period. If the patient drinks 232.0 mL, 300. mL, and 41 mL of water, what is the total volume of the fluid intake?
A) 573.0 mL
B) 573 mL
C) 570 mL
D) 600 mL
E) 57 mL
A) 573.0 mL
B) 573 mL
C) 570 mL
D) 600 mL
E) 57 mL

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
Convert 0.038 L to milliliters.
A) 3.8 mL
B) 38 mL
C) 380 mL
D) 3.8 102 mL
E) 3.8 105 mL
A) 3.8 mL
B) 38 mL
C) 380 mL
D) 3.8 102 mL
E) 3.8 105 mL

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
The number of significant figures in the measurement 5.40 105 kg is
A) one.
B) two.
C) three.
D) five.
E) eight.
A) one.
B) two.
C) three.
D) five.
E) eight.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
Using significant figures, what is the sum of 12.01 + 1011 + 0.113?
A) 1023.123
B) 1023.12
C) 1023
D) 1020
E) 1000
A) 1023.123
B) 1023.12
C) 1023
D) 1020
E) 1000

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
Measurements taken with ruler A and ruler B differ slightly. In what way do the measurements differ? 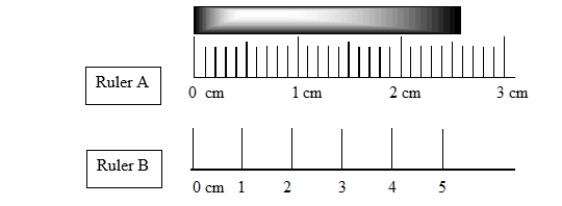
A) A measurement taken with ruler A has two more significant digits than ruler B
B) A measurement taken with ruler B has two more significant digits than ruler A
C) A measurement taken with ruler A has one more significant digit than ruler B
D) A measurement taken with ruler B has one more significant digit than ruler A
E) Measurements taken with ruler A and B are the same.

A) A measurement taken with ruler A has two more significant digits than ruler B
B) A measurement taken with ruler B has two more significant digits than ruler A
C) A measurement taken with ruler A has one more significant digit than ruler B
D) A measurement taken with ruler B has one more significant digit than ruler A
E) Measurements taken with ruler A and B are the same.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
To make this table, four different people took three measurements each of the distance between the chemistry building and the cafeteria on campus. Each person used a different measuring device and therefore arrived at a different set of measurements. The true distance is 152 meters. Which person is most accurate and precise? 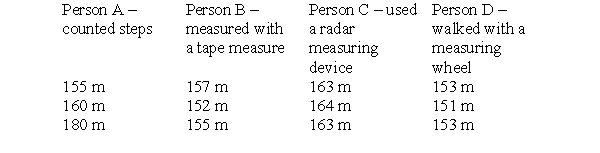
A) person A
B) person B
C) person C
D) person D
E) All are equally accurate and precise.

A) person A
B) person B
C) person C
D) person D
E) All are equally accurate and precise.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following unit conversions are useful when converting 312 mg to kilograms? 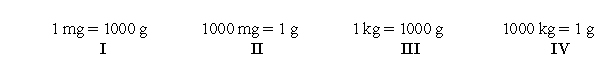
A) I and III
B) II and IV
C) I and IV
D) II and III
E) All of them are useful.
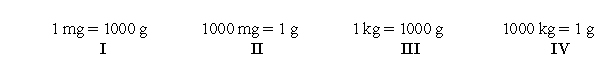
A) I and III
B) II and IV
C) I and IV
D) II and III
E) All of them are useful.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
Which statement comparing the accuracies and precisions of ruler A and ruler B is correct? 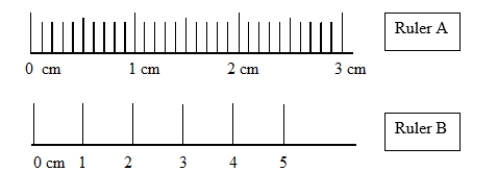
A) Ruler A is more precise and more accurate than ruler B
B) Ruler B is more accurate than ruler A, but less precise.
C) Ruler A is more accurate than ruler B, but less precise.
D) Ruler B is more precise and more accurate than ruler A
E) Ruler A is more precise than ruler B, but it is not possible to compare accuracy based on the information given.

A) Ruler A is more precise and more accurate than ruler B
B) Ruler B is more accurate than ruler A, but less precise.
C) Ruler A is more accurate than ruler B, but less precise.
D) Ruler B is more precise and more accurate than ruler A
E) Ruler A is more precise than ruler B, but it is not possible to compare accuracy based on the information given.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
The number of significant figures in the measurement of 0.004500 cm3 is
A) two
B) four
C) five
D) six
E) seven
A) two
B) four
C) five
D) six
E) seven

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is an exact number?
A) the number of milligrams in a gram
B) the number of meters in a kilometer
C) the number of micrometers in a centimeter
D) the number of cubic centimeters in a liter
E) All of the above
A) the number of milligrams in a gram
B) the number of meters in a kilometer
C) the number of micrometers in a centimeter
D) the number of cubic centimeters in a liter
E) All of the above

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
How many micrometers are there in 52.6 km?
A) 5.26 108 μm
B) 0.0526 μm
C) 5260 μm
D) 5.26 109 μm
E) 5.26 1010 μm
A) 5.26 108 μm
B) 0.0526 μm
C) 5260 μm
D) 5.26 109 μm
E) 5.26 1010 μm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
Tylenol is ordered for a child weighing 42 pounds at a dosage of 15 mg per kilogram of body weight. You need to determine how many mg of Tylenol should be administered to this child in a single dose. In order to answer this question, it is also necessary to use a conversion factor that must be looked up in a table (or have memorized). Which conversion factor is this? 
A) I
B) II
C) III
D) IV
E) Both III and IV

A) I
B) II
C) III
D) IV
E) Both III and IV

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
Tylenol is ordered for a child weighing 42 pounds at a dosage of 15 mg per kilogram of body weight. You need to determine how many mg of Tylenol should be administered to this child in a single dose. Which of the following equations is set up to find the answer to this problem?
A)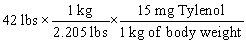
B)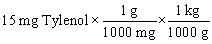
C)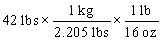
D)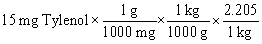
E)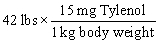
A)
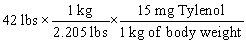
B)
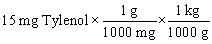
C)
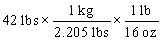
D)
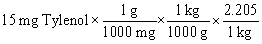
E)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
What is the density of substance with a mass of 10.6 g and a volume of 12.0 mL?
A) 0.883 g/mL
B) 1.4 g/mL
C) 22.6 g/mL
D) 1.13 mL/g
E) 127 gmL
A) 0.883 g/mL
B) 1.4 g/mL
C) 22.6 g/mL
D) 1.13 mL/g
E) 127 gmL

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following conversion factors are useful when converting 312 mg to kilograms? 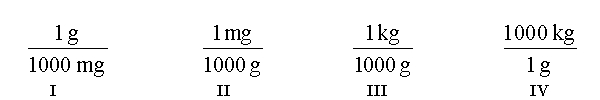
A) II and III
B) II and IV
C) I and IV
D) I and III
E) All of them are useful.
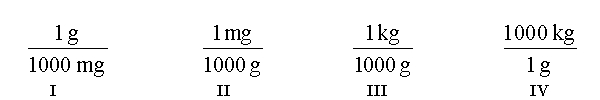
A) II and III
B) II and IV
C) I and IV
D) I and III
E) All of them are useful.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
How many mg of Tylenol should be administered to this child in a single dose?
A) 14 mg
B) 19 mg
C) 300 mg
D) 290 mg
E) 630 mg
A) 14 mg
B) 19 mg
C) 300 mg
D) 290 mg
E) 630 mg

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
The smallest bone on the body, the stirrup-shaped stapes found in the middle ear, has a typical length of less than 0.33 cm. How long is typical maximum length of the stapes in inches?
A) 7.7 in
B) 1 in
C) 0.84 in
D) 0.8 in
E) 0.13 in
A) 7.7 in
B) 1 in
C) 0.84 in
D) 0.8 in
E) 0.13 in

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
Tylenol is ordered for a child weighing 42 pounds at a dosage of 15 mg per kilogram of body weight. You need to determine how many mg of Tylenol should be administered to this child in a single dose. In order to answer this question, a conversion is used that is actually written within the body of the question. Which factor is this?
A) 42 pounds = 15 mg of Tylenol
B) 42 pounds = 1 kilogram of body weight
C) 15 mg of Tylenol = 1 kilogram of body weight
D) 15 mg of Tylenol = 1 pound of body weight
E) 1 pound = 1 kilogram of body weight
A) 42 pounds = 15 mg of Tylenol
B) 42 pounds = 1 kilogram of body weight
C) 15 mg of Tylenol = 1 kilogram of body weight
D) 15 mg of Tylenol = 1 pound of body weight
E) 1 pound = 1 kilogram of body weight

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following conversions are needed to convert 36.2 inches to centimeters? 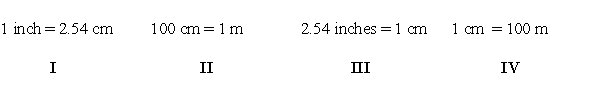
A) I
B) II
C) III
D) IV
E) I and II
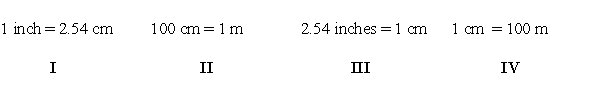
A) I
B) II
C) III
D) IV
E) I and II

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
Oil floats on water because oil is ______ than water.
A) heavier
B) less dense
C) lighter
D) denser
E) lower in volume
A) heavier
B) less dense
C) lighter
D) denser
E) lower in volume

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
Convert 312 mg to kilograms.
A) 3.12 104 kg
B) 3.12 kg
C) 0.312 kg
D) 3.12 105 kg
E) 3.12 108 kg
A) 3.12 104 kg
B) 3.12 kg
C) 0.312 kg
D) 3.12 105 kg
E) 3.12 108 kg

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Water has a density of 1.0 g/mL. What is the mass of 25 mL of water?
A) 0.25 g
B) 2.5 g
C) 25 g
D) 250 g
E) 25 kg
A) 0.25 g
B) 2.5 g
C) 25 g
D) 250 g
E) 25 kg

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
A patient weighs 78 kg. What is his weight in pounds?
A) 35 lb
B) 78 lb
C) 80 lb
D) 170 lb
E) 1.7 x 105 lb
A) 35 lb
B) 78 lb
C) 80 lb
D) 170 lb
E) 1.7 x 105 lb

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
A 1.0 102 Watt light bulb uses 6.0 103 J of energy per minute. How many Calories of energy does a 1.0 102 W light bulb use in one minute?
A) 1.4 Calories
B) 250 Calories
C) 1400 Calories
D) 6 x 103 Calories
E) 2.5 x 104 Calories
A) 1.4 Calories
B) 250 Calories
C) 1400 Calories
D) 6 x 103 Calories
E) 2.5 x 104 Calories

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following equations is set up to convert 312 mg to kilograms?
A)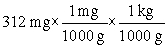
B)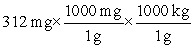
C)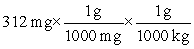
D)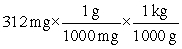
E)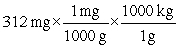
A)
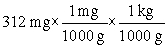
B)
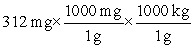
C)
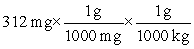
D)
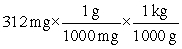
E)
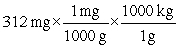

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
Tylenol is ordered for a child weighing 42 pounds at a dosage of 15 mg per kilogram of body weight. You need to determine how many mg of Tylenol should be administered to this child in a single dose. Which of the following units will be in the answer to this question (i.e., is requested)?
A) pounds of body weight
B) kilograms of body weight
C) milligrams of Tylenol
D) ounces of Tylenol
E) tablets of Tylenol
A) pounds of body weight
B) kilograms of body weight
C) milligrams of Tylenol
D) ounces of Tylenol
E) tablets of Tylenol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
A child's height is 36.2 inches. What is her height in centimeters?
A) 91.95 cm
B) 92 cm
C) 91.9 cm
D) 14.25 cm
E) 14.3 cm
A) 91.95 cm
B) 92 cm
C) 91.9 cm
D) 14.25 cm
E) 14.3 cm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
A medium apple provides about 80 Calories. How many calories are provided by the apple?
A) 0.0008 calories
B) 0.008 calories
C) 80 calories
D) 8000 calories
E) 8 104 calories
A) 0.0008 calories
B) 0.008 calories
C) 80 calories
D) 8000 calories
E) 8 104 calories

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following conversion factors are required to convert 36.2 inches to centimeters? 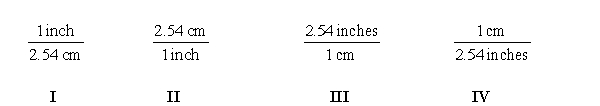
A) I
B) II
C) III
D) IV
E) None of them are required.
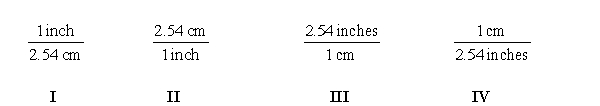
A) I
B) II
C) III
D) IV
E) None of them are required.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
A child's height is 36.2 inches and she wants to know her height in centimeters. In this problem, ______ is the supplied unit and ______ is the requested unit.
A) centimeters; inches
B) meters; inches
C) inches; centimeters
D) inches; meters
E) meters; centimeters
A) centimeters; inches
B) meters; inches
C) inches; centimeters
D) inches; meters
E) meters; centimeters

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
Tetracycline is a short acting antibiotic. It discolors developing teeth and so is not normally prescribed for children under 8 or pregnant women. An 11-year-old, 84-lb child is prescribed 35 mg/kg tetracycline per day for 10 days. What is the daily dose of tetracycline that should be administered to the child?
A) 5.3 mg
B) 53 mg
C) 1.3 g
D) 1.3 mg
E) 2.9 g
A) 5.3 mg
B) 53 mg
C) 1.3 g
D) 1.3 mg
E) 2.9 g

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


