Deck 16: Kinetics: Rates and Mechanisms of Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/89
Play
Full screen (f)
Deck 16: Kinetics: Rates and Mechanisms of Chemical Reactions
1
For the reaction A(g) + 2B(g) 2C(g) + 2D(g), the following data were collected at constant temperature. Determine the correct rate law for this reaction. ![<strong>For the reaction A(g) + 2B(g) \rightarrow 2C(g) + 2D(g), the following data were collected at constant temperature. Determine the correct rate law for this reaction. </strong> A) Rate = k[A] [B] B) Rate = k[A]<sup>2</sup> [B] C) Rate = k[A] [B]<sup>2</sup> D) Rate = k[A] E) Rate = k[A]<sup>3</sup>](https://storage.examlex.com/TB7799/11eb16b2_ffc7_b63c_984d_b5c06c221594_TB7799_00.jpg)
A) Rate = k[A] [B]
B) Rate = k[A]2 [B]
C) Rate = k[A] [B]2
D) Rate = k[A]
E) Rate = k[A]3
![<strong>For the reaction A(g) + 2B(g) \rightarrow 2C(g) + 2D(g), the following data were collected at constant temperature. Determine the correct rate law for this reaction. </strong> A) Rate = k[A] [B] B) Rate = k[A]<sup>2</sup> [B] C) Rate = k[A] [B]<sup>2</sup> D) Rate = k[A] E) Rate = k[A]<sup>3</sup>](https://storage.examlex.com/TB7799/11eb16b2_ffc7_b63c_984d_b5c06c221594_TB7799_00.jpg)
A) Rate = k[A] [B]
B) Rate = k[A]2 [B]
C) Rate = k[A] [B]2
D) Rate = k[A]
E) Rate = k[A]3
Rate = k[A]
2
Which one of the following sets of units is appropriate for a third-order rate constant?
A) s¯1
B) mol L¯1 s¯1
C) L mol¯1 s¯1
D) L2 mol¯2 s¯1
E) L3 mol¯3 s¯1
A) s¯1
B) mol L¯1 s¯1
C) L mol¯1 s¯1
D) L2 mol¯2 s¯1
E) L3 mol¯3 s¯1
L2 mol¯2 s¯1
3
A study of the decomposition reaction 3RS2 3R + 6S yields the initial rate data below. What is the rate constant for the reaction? 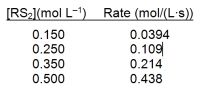
A) 0.0103 L mol¯1s¯1
B) 0.263 L mol¯1s¯1
C) 0.571 L mol¯1s¯1
D) 1.17 L mol¯1s¯1
E) 1.75 L mol¯1s¯1

A) 0.0103 L mol¯1s¯1
B) 0.263 L mol¯1s¯1
C) 0.571 L mol¯1s¯1
D) 1.17 L mol¯1s¯1
E) 1.75 L mol¯1s¯1
1.75 L mol¯1s¯1
4
Which of the following sets of units could be appropriate for a zero-order rate constant?
A) s¯1
B) L mol¯1 s¯1
C) L2 mol¯2 s¯1
D) L3 mol¯3 s¯1
E) mol L¯1 s¯1
A) s¯1
B) L mol¯1 s¯1
C) L2 mol¯2 s¯1
D) L3 mol¯3 s¯1
E) mol L¯1 s¯1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
5
Ammonium cyanate (NH4CNO) reacts to form urea (NH2CONH2). At 65 C, the rate constant, k, is 3.60 L mol¯1s¯1. What is the rate law for this reaction?
A) Rate = 3.60 L mol¯1s¯1[NH4CNO]
B) Rate = 3.60 L mol¯1s¯1[NH4CNO]2
C) Rate = 0.28 mol L¯1s¯1[NH4CNO]
D) Rate = 0.28 mol L¯1s¯1[NH4CNO]2
E) Rate = 3.60 L mol¯1s¯1[NH2CONH2]¯1
A) Rate = 3.60 L mol¯1s¯1[NH4CNO]
B) Rate = 3.60 L mol¯1s¯1[NH4CNO]2
C) Rate = 0.28 mol L¯1s¯1[NH4CNO]
D) Rate = 0.28 mol L¯1s¯1[NH4CNO]2
E) Rate = 3.60 L mol¯1s¯1[NH2CONH2]¯1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
6
Consider this reaction:
2NH3(g) N2(g) + 3H2(g) If the rate [H2]/ t is 0.030 mol L¯1 s¯1, then [NH3]/ t is:
A) -0.045 mol L¯1 s¯1
B) -0.030 mol L¯1 s¯1
C) -0.020 mol L¯1 s¯1
D) -0.010 mol L¯1 s¯1
E) None of these choices is correct.
2NH3(g) N2(g) + 3H2(g) If the rate [H2]/ t is 0.030 mol L¯1 s¯1, then [NH3]/ t is:
A) -0.045 mol L¯1 s¯1
B) -0.030 mol L¯1 s¯1
C) -0.020 mol L¯1 s¯1
D) -0.010 mol L¯1 s¯1
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
7
Consider the general reaction:
5Br¯(aq) + BrO3¯(aq) + 6H+(aq) 3Br2(aq) + 3H2O(aq)
For this reaction, the rate when expressed as [Br2]/ t is the same as:
A) - [H2O]/ t
B) 3 [BrO3¯]/ t
C) -5 [Br¯]/ t
D) -0.6 [Br¯]/ t
E) None of these choices is correct.
5Br¯(aq) + BrO3¯(aq) + 6H+(aq) 3Br2(aq) + 3H2O(aq)
For this reaction, the rate when expressed as [Br2]/ t is the same as:
A) - [H2O]/ t
B) 3 [BrO3¯]/ t
C) -5 [Br¯]/ t
D) -0.6 [Br¯]/ t
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
8
The rate constant for a reaction is 4.65 L mol¯1s¯1. What is the overall order of the reaction?
A) zero
B) first
C) second
D) third
E) More information is needed to determine the overall order.
A) zero
B) first
C) second
D) third
E) More information is needed to determine the overall order.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
9
When the reaction A B + C is studied, a plot 1/[A]t vs. time gives a straight line with a positive slope. What is the order of the reaction?
A) zero
B) first
C) second
D) third
E) More information is needed to determine the order.
A) zero
B) first
C) second
D) third
E) More information is needed to determine the order.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
10
The compound RX3 decomposes according to the equation 3RX3 R + R2X3 + 3X2
In an experiment the following data were collected for the decomposition at 100 C. What is the average rate of reaction over the entire experiment?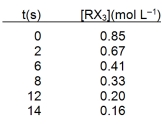
A) 0.011 mol L¯1s¯1
B) 0.019 mol L¯1s¯1
C) 0.044 mol L¯1s¯1
D) 0.049 mol L¯1s¯1
E) 0.069 mol L¯1s¯1
In an experiment the following data were collected for the decomposition at 100 C. What is the average rate of reaction over the entire experiment?

A) 0.011 mol L¯1s¯1
B) 0.019 mol L¯1s¯1
C) 0.044 mol L¯1s¯1
D) 0.049 mol L¯1s¯1
E) 0.069 mol L¯1s¯1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
11
Sulfur trioxide can undergo decomposition according to the equation
2SO3 2SO2 + O2
For this reaction, rate = -0 0.5 [SO3]/ t = k[SO3]2. If the reaction rate is 1.75 *10¯7 mol L¯1 min¯1 when the concentration of sulfur trioxide is 5.4 * 10¯3 mol L¯1, what is the value of the rate constant k?
A) 3.2 * 10¯5 L mol¯1 min¯1
B) 1.6 *10¯5 L mol¯1 min¯1
C) 6.0 * 10¯3 L mol¯1 min¯1
D) 3.0 * 10¯3 L mol¯1 min¯1
E) 1.6 * 10¯2 L mol¯1 min¯1
2SO3 2SO2 + O2
For this reaction, rate = -0 0.5 [SO3]/ t = k[SO3]2. If the reaction rate is 1.75 *10¯7 mol L¯1 min¯1 when the concentration of sulfur trioxide is 5.4 * 10¯3 mol L¯1, what is the value of the rate constant k?
A) 3.2 * 10¯5 L mol¯1 min¯1
B) 1.6 *10¯5 L mol¯1 min¯1
C) 6.0 * 10¯3 L mol¯1 min¯1
D) 3.0 * 10¯3 L mol¯1 min¯1
E) 1.6 * 10¯2 L mol¯1 min¯1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
12
Sulfuryl chloride, SO2Cl2(g), decomposes at high temperature to form SO2(g) and Cl2(g). The rate constant at a certain temperature is 4.68 *10¯5s¯1. What is the order of the reaction?
A) zero
B) first
C) second
D) third
E) More information is needed to determine the order.
A) zero
B) first
C) second
D) third
E) More information is needed to determine the order.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
13
When the reaction A B + C is studied, a plot of ln[A]t vs. time gives a straight line with a negative slope. What is the order of the reaction?
A) zero
B) first
C) second
D) third
E) More information is needed to determine the order.
A) zero
B) first
C) second
D) third
E) More information is needed to determine the order.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
14
Based on the initial rate data below, what is the value of the rate constant? 2NOBr(g) 2NO(g) + Br2(g) 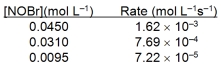
A) 0.0360 L mol¯1s¯1
B) 0.800 L mol¯1s¯1
C) 1.25 L mol¯1s¯1
D) 27.8 L mol¯1s¯1
E) 0.0360 s¯1

A) 0.0360 L mol¯1s¯1
B) 0.800 L mol¯1s¯1
C) 1.25 L mol¯1s¯1
D) 27.8 L mol¯1s¯1
E) 0.0360 s¯1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following sets of units is appropriate for a second-order rate constant?
A) s¯1
B) mol L¯1 s¯1
C) L mol¯1 s¯1
D) mol2 L¯2 s¯1
E) L2 mol¯2 s¯1
A) s¯1
B) mol L¯1 s¯1
C) L mol¯1 s¯1
D) mol2 L¯2 s¯1
E) L2 mol¯2 s¯1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
16
Sucrose decomposes to fructose and glucose in acid solution. When ln [sucrose] is plotted vs. time, a straight line with slope of -0.208 hr¯1 results. What is the rate law for the reaction?
A) Rate = 0.208 hr¯1 [sucrose]2
B) Rate = 0.208 hr¯1 [sucrose]
C) Rate = 0.0433 hr [sucrose]2
D) Rate = 0.0433 hr [sucrose]
E) Rate = 0.208 mol L¯1hr¯1 [sucrose]0
A) Rate = 0.208 hr¯1 [sucrose]2
B) Rate = 0.208 hr¯1 [sucrose]
C) Rate = 0.0433 hr [sucrose]2
D) Rate = 0.0433 hr [sucrose]
E) Rate = 0.208 mol L¯1hr¯1 [sucrose]0

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
17
Consider this reaction: 8A(g) + 5B(g) 8C(g) + 6D(g) If [C] is increasing at the rate of 4.0 mol L¯1s¯1, at what rate is [B] changing?
A) -0.40 mol L¯1s¯1
B) -2.5 mol L¯1s¯1
C) -4.0 mol L¯1s¯1
D) -6.4 mol L¯1s¯1
E) None of these choices is correct, since its rate of change must be positive.
A) -0.40 mol L¯1s¯1
B) -2.5 mol L¯1s¯1
C) -4.0 mol L¯1s¯1
D) -6.4 mol L¯1s¯1
E) None of these choices is correct, since its rate of change must be positive.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
18
?
A) 3.0
B) 6.0
C) 9.0
D) 18
E) 27
A) 3.0
B) 6.0
C) 9.0
D) 18
E) 27

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
19
For the reaction 3A(g) + 2B(g) 2C(g) + 2D(g), the following data were collected at constant temperature. Determine the correct rate law for this reaction. ![<strong>For the reaction 3A(g) + 2B(g) \rightarrow 2C(g) + 2D(g), the following data were collected at constant temperature. Determine the correct rate law for this reaction. </strong> A) Rate = k[A][B] B) Rate = k[A][B]<sup>2</sup> C) Rate = k[A]<sup>3</sup>[B]<sup>2</sup> D) Rate = k[A]<sup>1.5</sup>[B] E) Rate = k[A]<sup>2</sup>[B]](https://storage.examlex.com/TB7799/11eb16b2_ffc7_b63b_984d_171783f2c2ad_TB7799_00.jpg)
A) Rate = k[A][B]
B) Rate = k[A][B]2
C) Rate = k[A]3[B]2
D) Rate = k[A]1.5[B]
E) Rate = k[A]2[B]
![<strong>For the reaction 3A(g) + 2B(g) \rightarrow 2C(g) + 2D(g), the following data were collected at constant temperature. Determine the correct rate law for this reaction. </strong> A) Rate = k[A][B] B) Rate = k[A][B]<sup>2</sup> C) Rate = k[A]<sup>3</sup>[B]<sup>2</sup> D) Rate = k[A]<sup>1.5</sup>[B] E) Rate = k[A]<sup>2</sup>[B]](https://storage.examlex.com/TB7799/11eb16b2_ffc7_b63b_984d_171783f2c2ad_TB7799_00.jpg)
A) Rate = k[A][B]
B) Rate = k[A][B]2
C) Rate = k[A]3[B]2
D) Rate = k[A]1.5[B]
E) Rate = k[A]2[B]

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
20
For the reaction 2A + B + 2C D + E, the following initial rate data were collected at constant temperature. Determine the correct rate law for this reaction. All units are arbitrary. ![<strong>For the reaction 2A + B + 2C \rightarrow D + E, the following initial rate data were collected at constant temperature. Determine the correct rate law for this reaction. All units are arbitrary. </strong> A) Rate = k[A][B][C] B) Rate = k [A]<sup>2</sup>[B][C] C) Rate = k [A]<sup>2</sup>[B][C]¯<sup>1</sup> D) Rate = k [A][B]<sup>2</sup>[C]¯<sup>1</sup> E) None of these choices is correct.](https://storage.examlex.com/TB7799/11eb16b2_ffc7_dd4d_984d_8d6277d37ce7_TB7799_00.jpg)
A) Rate = k[A][B][C]
B) Rate = k [A]2[B][C]
C) Rate = k [A]2[B][C]¯1
D) Rate = k [A][B]2[C]¯1
E) None of these choices is correct.
![<strong>For the reaction 2A + B + 2C \rightarrow D + E, the following initial rate data were collected at constant temperature. Determine the correct rate law for this reaction. All units are arbitrary. </strong> A) Rate = k[A][B][C] B) Rate = k [A]<sup>2</sup>[B][C] C) Rate = k [A]<sup>2</sup>[B][C]¯<sup>1</sup> D) Rate = k [A][B]<sup>2</sup>[C]¯<sup>1</sup> E) None of these choices is correct.](https://storage.examlex.com/TB7799/11eb16b2_ffc7_dd4d_984d_8d6277d37ce7_TB7799_00.jpg)
A) Rate = k[A][B][C]
B) Rate = k [A]2[B][C]
C) Rate = k [A]2[B][C]¯1
D) Rate = k [A][B]2[C]¯1
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
21
The decomposition of SOCl2 is first-order in SOCl2. If the half-life for the reaction is 4.1 hr, how long would it take for the concentration of SOCl2 to drop from 0.36 M to 0.045 M?
A) 0.52 hr
B) 1.4 hr
C) 12 hr
D) 33 hr
E) > 40 hr
A) 0.52 hr
B) 1.4 hr
C) 12 hr
D) 33 hr
E) > 40 hr

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
22
A reactant R is being consumed in a first-order reaction. What fraction of the initial R is consumed in 4.0 half-lives?
A) 0.94
B) 0.87
C) 0.75
D) 0.13
E) 0.063
A) 0.94
B) 0.87
C) 0.75
D) 0.13
E) 0.063

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
23
Tetrafluoroethylene, C2F4, can be converted to octafluorocyclobutane which can be used as a refrigerant or an aerosol propellant. A plot of 1/[C2F4] vs. time gives a straight line with a slope of 0.0448 L mol¯1s¯1. What is the rate law for this reaction?
A) Rate = 0.0448 (L mol¯1s¯1)[C2F4]
B) Rate = 22.3 (mol L¯1s)[C2F4]
C) Rate = 0.0448 (L mol¯1s¯1)[C2F4]2
D) Rate = 22.3 (mol L¯1s)[C2F4]2
E) Rate = 0.0448 s¯1 [C2F4]
A) Rate = 0.0448 (L mol¯1s¯1)[C2F4]
B) Rate = 22.3 (mol L¯1s)[C2F4]
C) Rate = 0.0448 (L mol¯1s¯1)[C2F4]2
D) Rate = 22.3 (mol L¯1s)[C2F4]2
E) Rate = 0.0448 s¯1 [C2F4]

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
24
Cyclopropane is converted to propene in a first-order process. The rate constant is 5.4 * 10¯2 hr¯1. If the initial concentration of cyclopropane is 0.150 M, what will its concentration be after 22.0 hours?
A) 0.0457 M
B) 0.105 M
C) 0.127 M
D) 0.492 M
E) None of these choices is correct.
A) 0.0457 M
B) 0.105 M
C) 0.127 M
D) 0.492 M
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
25
A first-order reaction has a half-life of 20.0 minutes. Starting with 1.00 *1020 molecules of reactant at time t = 0, how many molecules remain unreacted after 100.0 minutes?
A) 1.00 * 104 molecules
B) 2.00 * 1019 molecules
C) 3.20 *1016 molecules
D) 5.00 * 1020 molecules
E) None of these choices is correct.
A) 1.00 * 104 molecules
B) 2.00 * 1019 molecules
C) 3.20 *1016 molecules
D) 5.00 * 1020 molecules
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
26
A reaction is first-order with respect to the reactant R. Which of the following plots will produce a straight line?
A) [R] vs. 1/time
B) 1/[R] vs. time
C) [R]2 vs. time
D) 1/[R]2 vs. time
E) ln[R] vs. time
A) [R] vs. 1/time
B) 1/[R] vs. time
C) [R]2 vs. time
D) 1/[R]2 vs. time
E) ln[R] vs. time

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
27
Carbon-14 is a radioactive isotope which decays with a half-life of 5730 years. What is the first-order rate constant for its decay, in units of years¯1?
A) 5.25 * 10¯5 years¯1
B) 1.21 * 10¯4 years¯1
C) 1.75 * 10¯4 years¯1
D) 3.49 * 10¯4 years¯1
E) 3.97 *103 years¯1
A) 5.25 * 10¯5 years¯1
B) 1.21 * 10¯4 years¯1
C) 1.75 * 10¯4 years¯1
D) 3.49 * 10¯4 years¯1
E) 3.97 *103 years¯1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
28
The rate law for the rearrangement of CH3NC to CH3CN at 800 K is Rate = (1300 s¯1)[CH3NC]. What is the half-life for this reaction?
A) 7.69 * 10¯4 s
B) 5.3* 10¯4 s
C) 1.9 * 10¯3 s
D) 520 s
E) 1920 s
A) 7.69 * 10¯4 s
B) 5.3* 10¯4 s
C) 1.9 * 10¯3 s
D) 520 s
E) 1920 s

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
29
The rate constant for the reaction 3A 4B is 6.00 *10¯3 L mol¯1min¯1. How long will it take the concentration of A to drop from 0.75 M to 0.25 M?
A) 2.2 * 10¯3 min
B) 5.5 * 10¯3 min
C) 180 min
D) 440 min
E) 5.0 * 102 min
A) 2.2 * 10¯3 min
B) 5.5 * 10¯3 min
C) 180 min
D) 440 min
E) 5.0 * 102 min

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
30
A gas-phase decomposition is first-order with respect to the reactant, R. If the initial concentration of R is 1.0 *¯4 mol L¯1 and the rate constant k = 1.08 *10¯6 s¯1, what concentration of R remains after 25 days?
A) 1.0 * 10¯3 mol L¯1
B) 1.0 *10¯4 mol L¯1
C) 9.6 * 10¯5 mol L¯1
D) 4.3 * 10¯5 mol L¯1
E) 9.7 *10¯6 mol L¯1
A) 1.0 * 10¯3 mol L¯1
B) 1.0 *10¯4 mol L¯1
C) 9.6 * 10¯5 mol L¯1
D) 4.3 * 10¯5 mol L¯1
E) 9.7 *10¯6 mol L¯1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
31
The rate law for the reaction 3A C is Rate = 4.36 *10¯2 L mol¯1 hr¯1[A]2 What is the half-life for the reaction if the initial concentration of A is 0.250 M?
A) 0.0109 hr
B) 0.0629 hr
C) 15.9 hr
D) 23.9 hr
E) 91.7 hr
A) 0.0109 hr
B) 0.0629 hr
C) 15.9 hr
D) 23.9 hr
E) 91.7 hr

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
32
The reaction CH3NC(g) CH3CN(g) is first-order with respect to methyl isocyanide, CH3NC. If it takes 10.3 minutes for exactly one quarter of the initial amount of methyl isocyanide to react, what is the rate constant in units of min¯1?
A) -0.135 min¯1
B) 0.0279 min¯1
C) 0.089 min¯1
D) 0.135 min¯1
E) 35.8 min¯1
A) -0.135 min¯1
B) 0.0279 min¯1
C) 0.089 min¯1
D) 0.135 min¯1
E) 35.8 min¯1

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
33
The active ingredient in an over-the-counter pain killer analgesic decomposes with a rate constant, k = 9.05 x 10¯4 day¯1. How many days does it take for 15% of the original ingredient to decompose?
A) 730 days
B) 414 days
C) 365 days
D) 180 days
E) 78 days
A) 730 days
B) 414 days
C) 365 days
D) 180 days
E) 78 days

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
34
A reaction is second-order with respect to the reactant R. Which of the following plots will produce a straight line?
A) [R] vs. 1/time
B) 1/[R] vs. time
C) [R]2 vs. time
D) 1/[R]2 vs. time
E) ln[R] vs. time
A) [R] vs. 1/time
B) 1/[R] vs. time
C) [R]2 vs. time
D) 1/[R]2 vs. time
E) ln[R] vs. time

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
35
The decomposition of hydrogen peroxide is a first-order process with a rate constant of 1.06 *10¯3 min¯1. How long will it take for the concentration of H2O2 to drop from 0.0200 M to 0.0120 M?
A) < 1 min
B) 7.55 min
C) 481 min
D) 4550 min
E) 31,400 min
A) < 1 min
B) 7.55 min
C) 481 min
D) 4550 min
E) 31,400 min

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
36
The rate law for the reaction 3A 2B is rate = k[A] with a rate constant of 0.0447 hr¯1. What is the half-life of the reaction?
A) 0.0224 hr
B) 0.0645 hr
C) 15.5 hr
D) 22.4 hr
E) 44.7 hr
A) 0.0224 hr
B) 0.0645 hr
C) 15.5 hr
D) 22.4 hr
E) 44.7 hr

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
37
The radioactive isotope tritium decays with a first-order rate constant k of 0.056 year¯1. What fraction of the tritium initially in a sample is still present 30 years later?
A) 0.19
B) 0.60
C) 0.15
D) 2.8 *10¯38
E) None of these choices is correct.
A) 0.19
B) 0.60
C) 0.15
D) 2.8 *10¯38
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
38
Dinitrogen tetraoxide, N2O4, decomposes to nitrogen dioxide, NO2, in a first-order process. If k = 2.5 * 103 s¯1 at -5 C and k = 3.5 * 104 s¯1 at 25 C, what is the activation energy for the decomposition?
A) 0.73 kJ/mol
B) 58 kJ/mol
C) 140 kJ/mol
D) 580 kJ/mol
E) > 1000 kJ/mol
A) 0.73 kJ/mol
B) 58 kJ/mol
C) 140 kJ/mol
D) 580 kJ/mol
E) > 1000 kJ/mol

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
39
Butadiene, C4H6 (used to make synthetic rubber and latex paints) reacts to C8H12 with a rate law of rate = 0.014 L/(mol·s) [C4H6]2. What will be the concentration of C4H6 after 3.0 hours if the initial concentration is 0.025 M?
A) 0.0052 M
B) 0.024 M
C) 43 M
D) 190 M
E) 0.0000 M
A) 0.0052 M
B) 0.024 M
C) 43 M
D) 190 M
E) 0.0000 M

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
40
The reaction X Y is first-order overall and first-order with respect to the reactant X. The result of doubling the initial concentration of X will be to
A) shorten the half-life of the reaction.
B) increase the rate constant of the reaction.
C) decrease the rate constant of the reaction.
D) shorten the time taken to reach equilibrium.
E) double the initial rate.
A) shorten the half-life of the reaction.
B) increase the rate constant of the reaction.
C) decrease the rate constant of the reaction.
D) shorten the time taken to reach equilibrium.
E) double the initial rate.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
41
A rate constant obeys the Arrhenius equation, the factor A being 2.2 *1013 s¯1 and the activation energy being 150. kJ mol¯1. What is the value of the rate constant at 227 C, in s¯1?
A) 2.1 *1013 s¯1
B) 6.7 * 10¯22 s¯1
C) 1.5 * 1011 s¯1
D) 4.7 *10¯3 s¯1
E) None of these choices is correct.
A) 2.1 *1013 s¯1
B) 6.7 * 10¯22 s¯1
C) 1.5 * 1011 s¯1
D) 4.7 *10¯3 s¯1
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
42
If the activation energy of a reaction decreases by 10.0 kJ/mol, from 100.0 to 90.0 kJ/mol, what effect will this have on the rate of reaction at 298K?
A) The rate will increase, by a factor of more than 50.
B) The rate will decrease, by a factor of more than 50.
C) The rate will increase, by a factor of less than 50.
D) The rate will decrease, by a factor of less than 50.
E) The rate will not change unless temperature changes.
A) The rate will increase, by a factor of more than 50.
B) The rate will decrease, by a factor of more than 50.
C) The rate will increase, by a factor of less than 50.
D) The rate will decrease, by a factor of less than 50.
E) The rate will not change unless temperature changes.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
43
What is the molecularity of the following elementary reaction? 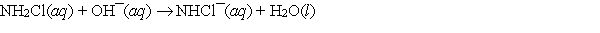
A) unimolecular
B) bimolecular
C) termolecular
D) tetramolecular
E) Need to know the reaction order before molecularity can be determined.
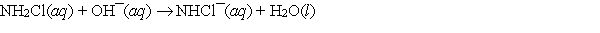
A) unimolecular
B) bimolecular
C) termolecular
D) tetramolecular
E) Need to know the reaction order before molecularity can be determined.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
44
A boiled egg can be cooked at 100.0 C in exactly 5 minutes. At an altitude of around 2000 m where the boiling point of water is 93.0 C, it takes exactly 7.5 minutes to cook the egg to the same amount. What is the activation energy for the reaction involved when an egg is boiled?
A) 0.5 kJ/mol
B) 4.5 kJ/mol
C) 7.9 kJ/mol
D) 66 kJ/mol
E) >100 kJ/mol
A) 0.5 kJ/mol
B) 4.5 kJ/mol
C) 7.9 kJ/mol
D) 66 kJ/mol
E) >100 kJ/mol

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
45
When a catalyst is added to a reaction mixture, it
A) increases the rate of collisions between reactant molecules.
B) provides reactant molecules with more energy.
C) slows down the rate of the back reaction.
D) provides a new pathway (mechanism) for the reaction.
E) None of these choices is correct.
A) increases the rate of collisions between reactant molecules.
B) provides reactant molecules with more energy.
C) slows down the rate of the back reaction.
D) provides a new pathway (mechanism) for the reaction.
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
46
A catalyst accelerates a reaction because
A) it increases the number of molecules with energy equal to or greater than the activation energy.
B) it lowers the activation energy for the reaction.
C) it increases the number of collisions between molecules.
D) it increases the temperature of the molecules in the reaction.
E) it supplies energy to reactant molecules.
A) it increases the number of molecules with energy equal to or greater than the activation energy.
B) it lowers the activation energy for the reaction.
C) it increases the number of collisions between molecules.
D) it increases the temperature of the molecules in the reaction.
E) it supplies energy to reactant molecules.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
47
Ammonia will react with oxygen in the presence of a copper catalyst to form nitrogen and water. From 164.5 C to 179.0 C, the rate constant increases by a factor of 4.27. What is the activation energy of this oxidation reaction?
A) 24.5 kJ/mol
B) 165 kJ/mol
C) 242 kJ/mol
D) 1630 kJ/mol
E) > 104 kJ/mol
A) 24.5 kJ/mol
B) 165 kJ/mol
C) 242 kJ/mol
D) 1630 kJ/mol
E) > 104 kJ/mol

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
48
(p. Various sections) The gas-phase reaction ![<strong>(p. Various sections) The gas-phase reaction has been studied in a closed vessel, and the rate equation was found to be: Rate = - \Delta [CH<sub>3</sub>NC]/ \Delta t = k[CH<sub>3</sub>NC]. Which one of the following actions is least likely to cause a change in the rate of the reaction?</strong> A) lowering the temperature B) adding a catalyst C) using a larger initial amount of CH<sub>3</sub>NC in the same vessel D) using a bigger vessel, but the same initial amount of CH<sub>3</sub>NC E) continuously removing CH<sub>3</sub>CN as it is formed](https://storage.examlex.com/TB7799/11eb16b2_ffc9_d827_984d_31a3a0ace6e5_TB7799_11.jpg) has been studied in a closed vessel, and the rate equation was found to be: Rate = - [CH3NC]/ t = k[CH3NC]. Which one of the following actions is least likely to cause a change in the rate of the reaction?
has been studied in a closed vessel, and the rate equation was found to be: Rate = - [CH3NC]/ t = k[CH3NC]. Which one of the following actions is least likely to cause a change in the rate of the reaction?
A) lowering the temperature
B) adding a catalyst
C) using a larger initial amount of CH3NC in the same vessel
D) using a bigger vessel, but the same initial amount of CH3NC
E) continuously removing CH3CN as it is formed
![<strong>(p. Various sections) The gas-phase reaction has been studied in a closed vessel, and the rate equation was found to be: Rate = - \Delta [CH<sub>3</sub>NC]/ \Delta t = k[CH<sub>3</sub>NC]. Which one of the following actions is least likely to cause a change in the rate of the reaction?</strong> A) lowering the temperature B) adding a catalyst C) using a larger initial amount of CH<sub>3</sub>NC in the same vessel D) using a bigger vessel, but the same initial amount of CH<sub>3</sub>NC E) continuously removing CH<sub>3</sub>CN as it is formed](https://storage.examlex.com/TB7799/11eb16b2_ffc9_d827_984d_31a3a0ace6e5_TB7799_11.jpg) has been studied in a closed vessel, and the rate equation was found to be: Rate = - [CH3NC]/ t = k[CH3NC]. Which one of the following actions is least likely to cause a change in the rate of the reaction?
has been studied in a closed vessel, and the rate equation was found to be: Rate = - [CH3NC]/ t = k[CH3NC]. Which one of the following actions is least likely to cause a change in the rate of the reaction?A) lowering the temperature
B) adding a catalyst
C) using a larger initial amount of CH3NC in the same vessel
D) using a bigger vessel, but the same initial amount of CH3NC
E) continuously removing CH3CN as it is formed

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
49
The kinetics of the decomposition of dinitrogen pentaoxide is studied at 50 C and at 75 C. Which of the following statements concerning the studies is correct?
A) The rate at 75 C will be greater than the rate at 50 C because the activation energy will be lower at 75 C than at 50 C.
B) The rate at 75 C will be greater than the rate at 50 C because the activation energy will be higher at 75 C than at 50 C.
C) The rate at 75 C will be less than the rate at 50 C because the molecules at higher speeds do not interact as well as those at lower speeds.
D) The rate at 75 C will be greater than at 50 C because the concentration of a gas increases with increasing temperature.
E) The rate at 75 C will be greater than the rate at 50 C because the number of molecules with enough energy to react increases with increasing temperature.
A) The rate at 75 C will be greater than the rate at 50 C because the activation energy will be lower at 75 C than at 50 C.
B) The rate at 75 C will be greater than the rate at 50 C because the activation energy will be higher at 75 C than at 50 C.
C) The rate at 75 C will be less than the rate at 50 C because the molecules at higher speeds do not interact as well as those at lower speeds.
D) The rate at 75 C will be greater than at 50 C because the concentration of a gas increases with increasing temperature.
E) The rate at 75 C will be greater than the rate at 50 C because the number of molecules with enough energy to react increases with increasing temperature.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
50
In going from room temperature (25.0 C) to 10 C above room temperature, the rate of a reaction doubles. Calculate the activation energy for the reaction.
A) 157.2 kJ/mol
B) 103.8 kJ/mol
C) 52.9 kJ/mol
D) 6.4 kJ/mol
E) <1 kJ/mol
A) 157.2 kJ/mol
B) 103.8 kJ/mol
C) 52.9 kJ/mol
D) 6.4 kJ/mol
E) <1 kJ/mol

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. ![<strong>Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. Which of the following rate laws is consistent with the mechanism?</strong> A) Rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>]<sup>2</sup>[Br¯] B) Rate = k [H<sub>2</sub>O<sup>+</sup> <sup> </sup> OH][Br¯] C) Rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>][Br¯] D) Rate = k[HOBr][H<sup>+</sup>][Br¯][H<sub>2</sub>O<sub>2</sub>] E) Rate = k[Br¯]](https://storage.examlex.com/TB7799/11eb16b2_ffc9_b115_984d_17e69fe2b888_TB7799_00.jpg) Which of the following rate laws is consistent with the mechanism?
Which of the following rate laws is consistent with the mechanism?
A) Rate = k[H2O2][H+]2[Br¯]
B) Rate = k [H2O+
![<strong>Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. Which of the following rate laws is consistent with the mechanism?</strong> A) Rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>]<sup>2</sup>[Br¯] B) Rate = k [H<sub>2</sub>O<sup>+</sup> <sup> </sup> OH][Br¯] C) Rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>][Br¯] D) Rate = k[HOBr][H<sup>+</sup>][Br¯][H<sub>2</sub>O<sub>2</sub>] E) Rate = k[Br¯]](https://storage.examlex.com/TB7799/11eb16b2_ffc9_b116_984d_1565a733b8c1_TB7799_11.jpg) OH][Br¯]
OH][Br¯]
C) Rate = k[H2O2][H+][Br¯]
D) Rate = k[HOBr][H+][Br¯][H2O2]
E) Rate = k[Br¯]
![<strong>Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. Which of the following rate laws is consistent with the mechanism?</strong> A) Rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>]<sup>2</sup>[Br¯] B) Rate = k [H<sub>2</sub>O<sup>+</sup> <sup> </sup> OH][Br¯] C) Rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>][Br¯] D) Rate = k[HOBr][H<sup>+</sup>][Br¯][H<sub>2</sub>O<sub>2</sub>] E) Rate = k[Br¯]](https://storage.examlex.com/TB7799/11eb16b2_ffc9_b115_984d_17e69fe2b888_TB7799_00.jpg) Which of the following rate laws is consistent with the mechanism?
Which of the following rate laws is consistent with the mechanism?A) Rate = k[H2O2][H+]2[Br¯]
B) Rate = k [H2O+
![<strong>Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. Which of the following rate laws is consistent with the mechanism?</strong> A) Rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>]<sup>2</sup>[Br¯] B) Rate = k [H<sub>2</sub>O<sup>+</sup> <sup> </sup> OH][Br¯] C) Rate = k[H<sub>2</sub>O<sub>2</sub>][H<sup>+</sup>][Br¯] D) Rate = k[HOBr][H<sup>+</sup>][Br¯][H<sub>2</sub>O<sub>2</sub>] E) Rate = k[Br¯]](https://storage.examlex.com/TB7799/11eb16b2_ffc9_b116_984d_1565a733b8c1_TB7799_11.jpg) OH][Br¯]
OH][Br¯]C) Rate = k[H2O2][H+][Br¯]
D) Rate = k[HOBr][H+][Br¯][H2O2]
E) Rate = k[Br¯]

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
52
The decomposition of dinitrogen pentaoxide to nitrogen dioxide and oxygen follows first-order kinetics and has an activation energy of 102 kJ/mol. By what factor will the fraction of collisions with energy greater than or equal to the activation energy increase if the reaction temperature goes from 30 C to 60 C?
A) 1.00
B) 1.10
C) 2.00
D) 4.00
E) 38.4
A) 1.00
B) 1.10
C) 2.00
D) 4.00
E) 38.4

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following affects the activation energy of a reaction?
A) temperature of the reactants
B) concentrations of reactants
C) presence of a catalyst
D) surface area of reactants
E) reaction progress
A) temperature of the reactants
B) concentrations of reactants
C) presence of a catalyst
D) surface area of reactants
E) reaction progress

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution. 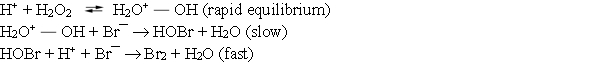 What is the overall reaction equation for this process?
What is the overall reaction equation for this process?
A) 2H2O+
 OH + 2Br¯ H2O2 + Br2 + 2H2O
OH + 2Br¯ H2O2 + Br2 + 2H2O
B) 2H+ + 2Br¯ + H2O2 Br2 + 2H2O
C) 2H+ + H2O2 + Br¯ + HOBr H2O+
 OH + Br2 + H2O
OH + Br2 + H2O
D) H2O+
 OH + Br¯ + H+ Br2 + H2O
OH + Br¯ + H+ Br2 + H2O
E) None of these choices is correct.
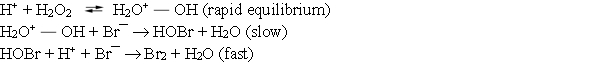 What is the overall reaction equation for this process?
What is the overall reaction equation for this process?A) 2H2O+
 OH + 2Br¯ H2O2 + Br2 + 2H2O
OH + 2Br¯ H2O2 + Br2 + 2H2OB) 2H+ + 2Br¯ + H2O2 Br2 + 2H2O
C) 2H+ + H2O2 + Br¯ + HOBr H2O+
 OH + Br2 + H2O
OH + Br2 + H2OD) H2O+
 OH + Br¯ + H+ Br2 + H2O
OH + Br¯ + H+ Br2 + H2OE) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
55
A reaction has an activation energy of 195.0 kJ/mol. When the temperature is increased from 200. C to 220. C, the rate constant will increase by a factor of:
A) 1.1
B) 4.3 *104
C) 3.2
D) 7.5
E) None of these choices is correct.
A) 1.1
B) 4.3 *104
C) 3.2
D) 7.5
E) None of these choices is correct.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
56
An increase in temperature increases the reaction rate because
A) a greater fraction of the collisions have the correct orientation of molecules.
B) the activation energy of the reaction will increase.
C) the activation energy of the reaction will decrease.
D) temperature acts as a catalyst in chemical reactions.
E) more collisions will have enough energy to exceed the activation energy.
A) a greater fraction of the collisions have the correct orientation of molecules.
B) the activation energy of the reaction will increase.
C) the activation energy of the reaction will decrease.
D) temperature acts as a catalyst in chemical reactions.
E) more collisions will have enough energy to exceed the activation energy.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
57
In the gas phase at 500. C, cyclopropane reacts to form propene in a first-order reaction. The figure below shows the concentration of cyclopropane plotted versus time. Use the graph to calculate approximate values of
A) the rate of the reaction, 600. seconds after the start.
B) the half-life of the reaction, t1/2.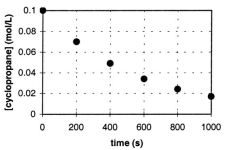
A) the rate of the reaction, 600. seconds after the start.
B) the half-life of the reaction, t1/2.


Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
58
In an exothermic reaction,
A) the forward reaction is slower than the reverse reaction.
B) the reaction rate will speed up with time.
C) the collision energy of the reactants will be greater than that of the products.
D) the forward reaction will have a lower activation energy than the reverse reaction.
E) the activation energy will change as the reaction progresses.
A) the forward reaction is slower than the reverse reaction.
B) the reaction rate will speed up with time.
C) the collision energy of the reactants will be greater than that of the products.
D) the forward reaction will have a lower activation energy than the reverse reaction.
E) the activation energy will change as the reaction progresses.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
59
The decomposition of dinitrogen pentaoxide has an activation energy of 102 kJ/mol and H rxn = + 55 kJ/mol. What is the activation energy for the reverse reaction?
A) 27 kJ/mol
B) 47 kJ/mol
C) 55 kJ/mol
D) 102 kJ/mol
E) More information is needed, since this is a Hess's law calculation.
A) 27 kJ/mol
B) 47 kJ/mol
C) 55 kJ/mol
D) 102 kJ/mol
E) More information is needed, since this is a Hess's law calculation.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
60
Reaction intermediates differ from activated complexes in that
A) they are stable molecules with normal bonds and are frequently isolated.
B) they are molecules with normal bonds rather than partial bonds and can occasionally be isolated.
C) they are intermediate structures which have characteristics of both reactants and products.
D) they are unstable and can never be isolated.
E) all reactions involve reaction intermediates, but not all have activated complexes.
A) they are stable molecules with normal bonds and are frequently isolated.
B) they are molecules with normal bonds rather than partial bonds and can occasionally be isolated.
C) they are intermediate structures which have characteristics of both reactants and products.
D) they are unstable and can never be isolated.
E) all reactions involve reaction intermediates, but not all have activated complexes.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
61
For each of the following terms/concepts, give a brief explanation or definition. Where possible, use examples.
A) order of a reaction
B) elementary reaction
C) reaction intermediate
A) order of a reaction
B) elementary reaction
C) reaction intermediate

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
62
Briefly outline the key arguments in the collision theory of reaction rates for the elementary reaction 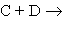 products. Show that this theory predicts a second-order rate law, and how it predicts the form of the rate constant k.
products. Show that this theory predicts a second-order rate law, and how it predicts the form of the rate constant k.
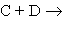 products. Show that this theory predicts a second-order rate law, and how it predicts the form of the rate constant k.
products. Show that this theory predicts a second-order rate law, and how it predicts the form of the rate constant k.
Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
63
The units of the rate constant depend on the order of the reaction.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
64
The rate law cannot be predicted from the stoichiometry of a reaction.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
65
Briefly list the features/properties common to all catalysts and how they work. Draw a labeled reaction energy diagram as part of your answer.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
66
An elementary reaction is a simple, one-step process.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
67
The elementary reaction HBr(g) + Br(g) H(g) + Br2(g) is endothermic.
A) Would you expect the rate constant for the back reaction to be smaller or larger than that for the forward reaction? Explain, briefly.
B) Draw a fully-labeled reaction energy diagram for this reaction, showing the locations of the reactants, products and transition state.
A) Would you expect the rate constant for the back reaction to be smaller or larger than that for the forward reaction? Explain, briefly.
B) Draw a fully-labeled reaction energy diagram for this reaction, showing the locations of the reactants, products and transition state.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
68
The gas-phase conversion of 1,3-butadiene to 1,5-cyclooctadiene, 2C4H6 C8H12, was studied, providing data for the plot shown in the graph, of 1/[butadiene] versus time.
A) Explain how this plot confirms that the reaction is second order.
B) Calculate the second-order rate constant, k.
C) Determine the initial concentration of 1,3-butadiene in this experiment.![<strong>The gas-phase conversion of 1,3-butadiene to 1,5-cyclooctadiene, 2C<sub>4</sub>H<sub>6</sub> \rightarrow C<sub>8</sub>H<sub>12</sub>, was studied, providing data for the plot shown in the graph, of 1/[butadiene] versus time.</strong> A) Explain how this plot confirms that the reaction is second order. B) Calculate the second-order rate constant, k. C) Determine the initial concentration of 1,3-butadiene in this experiment.](https://storage.examlex.com/TB7799/11eb16b2_ffca_264a_984d_31c229c16cf2_TB7799_00.jpg)
A) Explain how this plot confirms that the reaction is second order.
B) Calculate the second-order rate constant, k.
C) Determine the initial concentration of 1,3-butadiene in this experiment.
![<strong>The gas-phase conversion of 1,3-butadiene to 1,5-cyclooctadiene, 2C<sub>4</sub>H<sub>6</sub> \rightarrow C<sub>8</sub>H<sub>12</sub>, was studied, providing data for the plot shown in the graph, of 1/[butadiene] versus time.</strong> A) Explain how this plot confirms that the reaction is second order. B) Calculate the second-order rate constant, k. C) Determine the initial concentration of 1,3-butadiene in this experiment.](https://storage.examlex.com/TB7799/11eb16b2_ffca_264a_984d_31c229c16cf2_TB7799_00.jpg)

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
69
Is a bimolecular reaction necessarily second-order? Is a second-order reaction necessarily bimolecular? Answer, with explanations and clarifications.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
70
The half-life of a second-order reaction does not depend on the initial concentration of reactant.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
71
You are studying the rate of the reaction 2A B and have obtained measurements of the concentration of A at times t = 100, 200, 300, ......, 1000 seconds from the start of the reaction. Carefully describe how you would plot a graph and use it to
A) prove that the reaction is second-order with respect to
A.
B) determine the second-order rate constant k.
A) prove that the reaction is second-order with respect to
A.
B) determine the second-order rate constant k.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
72
In the collision theory of reaction rates, the rate constant for a bimolecular reaction can be written as k = z · p · exp 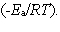 In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:
In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:
A) z
B) p
C) exp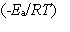
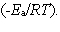 In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:
In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:A) z
B) p
C) exp

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
73
You are required to determine the energy of activation (Ea) of a reaction. Briefly describe the experimental measurements you would make and how you would obtain the activation energy from a suitable linear plot of the experimental data.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
74
In the gas phase at 500. C, cyclopropane reacts to form propene in a first-order reaction. The figure shows the natural logarithm of the concentration of cyclopropane (in mol/L) plotted versus time. 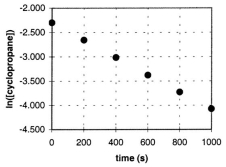 a. Explain how this plot confirms that the reaction is first order.
a. Explain how this plot confirms that the reaction is first order.
B) Calculate the first-order rate constant, k.
C) Determine the initial concentration of cyclopropane in this experiment.
 a. Explain how this plot confirms that the reaction is first order.
a. Explain how this plot confirms that the reaction is first order.B) Calculate the first-order rate constant, k.
C) Determine the initial concentration of cyclopropane in this experiment.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
75
A chemical reaction of the general type A 2B is first-order, with a rate constant of 1.52 *10¯4 s¯1.
A) Calculate the half-life of A.
B) Assuming the initial concentration of A is 0.067 mol L¯1, calculate the time needed for the concentration to fall to 0.010 mol L¯1.
A) Calculate the half-life of A.
B) Assuming the initial concentration of A is 0.067 mol L¯1, calculate the time needed for the concentration to fall to 0.010 mol L¯1.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
76
The units of the rate of reaction depend on the order of the reaction.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
77
The greater the energy of activation, Ea, the faster will be the reaction.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the general gas-phase reaction of a molecular substance, A:
1. At very low pressures many such reactions occur by the following mechanism:
At very low pressures many such reactions occur by the following mechanism:
2.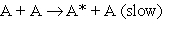 3.
3. 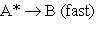 (A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
(A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
A) Which of the three reactions above is/are elementary?
B) Where appropriate, identify the molecularity of the reactions.
C) Show that the proposed mechanism is consistent with reaction 1, the observed reaction.
D) Given the mechanism above, suggest a likely rate law for reaction (1).
1.
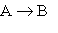 At very low pressures many such reactions occur by the following mechanism:
At very low pressures many such reactions occur by the following mechanism:2.
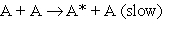 3.
3. 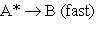 (A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
(A* represents a molecule with sufficient energy to overcome the activation energy barrier.)A) Which of the three reactions above is/are elementary?
B) Where appropriate, identify the molecularity of the reactions.
C) Show that the proposed mechanism is consistent with reaction 1, the observed reaction.
D) Given the mechanism above, suggest a likely rate law for reaction (1).

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
79
According to the collision theory of reaction rates, what are the three requirements which must be met before an elementary reaction between two molecules can occur?

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck
80
The half-life of a first-order reaction does not depend on the initial concentration of reactant.

Unlock Deck
Unlock for access to all 89 flashcards in this deck.
Unlock Deck
k this deck


