Deck 6: Intermolecular Forces Attractions Between Particles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/87
Play
Full screen (f)
Deck 6: Intermolecular Forces Attractions Between Particles
1
The relative energies strengths) of the intermolecular forces present in each of five different pure substances are shown in the figure below.Which substance has the highest melting point? 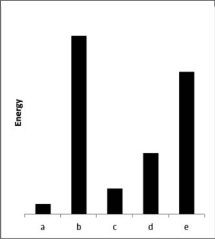
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e
b
2
Ion-dipole forces always require
A)an ion and a water molecule.
B)a cation and a water molecule.
C)an anion and a polar molecule.
D)an ion and a polar molecule.
E)a polar and a nonpolar molecule.
A)an ion and a water molecule.
B)a cation and a water molecule.
C)an anion and a polar molecule.
D)an ion and a polar molecule.
E)a polar and a nonpolar molecule.
an ion and a polar molecule.
3
Which molecule below exhibits the weakest dispersion forces?
A)O2
B)P4
C)Br2
D)I2
E)Xe
A)O2
B)P4
C)Br2
D)I2
E)Xe
O2
4
Which compound below has the highest boiling point?
A)CCl4
B)CI4
C)CF4
D)CH4
E)CBr4
A)CCl4
B)CI4
C)CF4
D)CH4
E)CBr4

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
5
Arrange the following compounds in order of increasing dispersion interactions: CCl4, CH4, CF4.
A)CH4 CF4 CCL4
B)CCL4 CH4 CF4
C)CF4 CH4 CCL4
D)CCL4 CF4 CH4
E)CH4 CCL4 CF4
A)CH4 CF4 CCL4
B)CCL4 CH4 CF4
C)CF4 CH4 CCL4
D)CCL4 CF4 CH4
E)CH4 CCL4 CF4

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement below regarding London dispersion forces is FALSE?
A)Electrons in one molecule can experience attractions to nuclei in a neighboring nonbonded molecule.
B)Temporary dipoles can arise when electrons are temporarily asymmetrically redistributed around the nucleus of an atom or within a bond.
C)As electron cloud size increases, the strength of London dispersion forces increases.
D)The dipole moments associated with London dispersion forces are long-lasting.
E)Temporary dipoles can induce dipoles in other particles.
A)Electrons in one molecule can experience attractions to nuclei in a neighboring nonbonded molecule.
B)Temporary dipoles can arise when electrons are temporarily asymmetrically redistributed around the nucleus of an atom or within a bond.
C)As electron cloud size increases, the strength of London dispersion forces increases.
D)The dipole moments associated with London dispersion forces are long-lasting.
E)Temporary dipoles can induce dipoles in other particles.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
7
The relative energies strengths) of the intermolecular forces present in each of five different pure substances are shown in the figure below.Which substance has the lowest melting point? 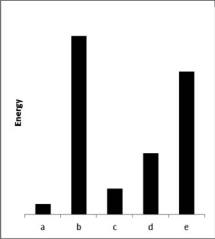
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
8
Polarizability refers to
A)the ease with which the electron cloud of an atom or molecule can be distorted.
B)the magnitude of the dipole moment of a molecule.
C)the ease with which a hydrogen bond can form.
D)the perturbation in electron density because of hydrogen bonding.
E)the ability to transmit polarized light.
A)the ease with which the electron cloud of an atom or molecule can be distorted.
B)the magnitude of the dipole moment of a molecule.
C)the ease with which a hydrogen bond can form.
D)the perturbation in electron density because of hydrogen bonding.
E)the ability to transmit polarized light.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
9
Which molecule below has the highest polarizability?
A)N2
B)O2
C)F2
D)Br2
E)I2
A)N2
B)O2
C)F2
D)Br2
E)I2

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
10
The relative energies strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below.Which substance is most likely to be a solid at room temperature? 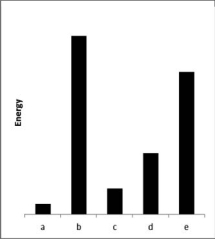
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
11
The relative energies (strengths) of the intermolecular forces between five different substances are shown in the figure below.Which substance has the highest boiling point?
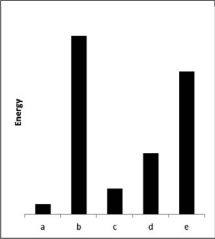
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
12
Which type of intermolecular interaction exists for all compounds?
A)ion-ion
B)dipole-dipole
C)London dispersion
D)hydrogen bonding
E)dipole-induced dipole
A)ion-ion
B)dipole-dipole
C)London dispersion
D)hydrogen bonding
E)dipole-induced dipole

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
13
The relative energies strengths) of the intermolecular forces between five different substances are shown in the figure below.Which substance has the lowest boiling point? 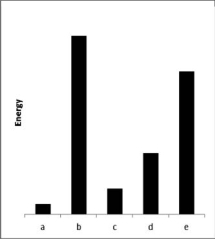
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
14
Which statement below is FALSE?
A)The more electrons in an atom, the more likely it is that they will be asymmetrically distributed.
B)The more electrons in an atom, the stronger the London dispersion forces it can experience.
C)Larger atoms may experience larger temporary dipoles.
D)Atoms of elements lower in a group of the periodic table probably experience larger London dispersion forces.
E)As the strength of temporary dipoles decreases within a substance, the boiling point increases.
A)The more electrons in an atom, the more likely it is that they will be asymmetrically distributed.
B)The more electrons in an atom, the stronger the London dispersion forces it can experience.
C)Larger atoms may experience larger temporary dipoles.
D)Atoms of elements lower in a group of the periodic table probably experience larger London dispersion forces.
E)As the strength of temporary dipoles decreases within a substance, the boiling point increases.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
15
Which straight-chain alkane below has the highest boiling point?
A)CH4
B)C6H12
C)C8H18
D)C2H6
E)C4H10
A)CH4
B)C6H12
C)C8H18
D)C2H6
E)C4H10

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
16
Which molecule below exhibits the greatest dispersion forces?
A)CH3CH3
B)CH3CH2CH3
C)C2H2
D)CH4
E)CH3CH2CH2CH3
A)CH3CH3
B)CH3CH2CH3
C)C2H2
D)CH4
E)CH3CH2CH2CH3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
17
Dispersion forces are due to
A)permanent dipoles.
B)temporary dipoles.
C)hydrogen bonding.
D)ionic interactions.
E)protons.
A)permanent dipoles.
B)temporary dipoles.
C)hydrogen bonding.
D)ionic interactions.
E)protons.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
18
The relative energies strengths) of the intermolecular forces present in each of four different pure substances are shown in the figure below.Which substance is most likely to be a gas at room temperature? 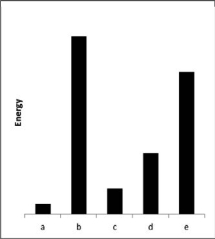
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
19
Viscosity is a measure of a substance's
A)ability to resist changes in its surface area.
B)resistance to flow.
C)surface tension.
D)compressibility.
E)color.
A)ability to resist changes in its surface area.
B)resistance to flow.
C)surface tension.
D)compressibility.
E)color.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
20
Which straight-chain alkane below would you predict to be the most viscous? All are liquids exhibiting the general bonding pattern CH3-(CH2 )n-CH3 .
A)C12H26
B)C10H22
C)C5H12
D)C6H14
E)C9H20
A)C12H26
B)C10H22
C)C5H12
D)C6H14
E)C9H20

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
21
Which compound below will exhibit hydrogen bonding with itself in the liquid state?
A)CH3OCH3
B)CH3COCH3
C)CH3CH2NH2
D)H2CO
E)CH3F
A)CH3OCH3
B)CH3COCH3
C)CH3CH2NH2
D)H2CO
E)CH3F

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
22
When two liquids mix completely in all proportions, they are
A)soluble.
B)miscible.
C)immiscible.
D)solvated.
E)ubiquitous.
A)soluble.
B)miscible.
C)immiscible.
D)solvated.
E)ubiquitous.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
23
The dipole moments of HCl and of HBr are 1.08 D and 0.82 D, respectively.The boiling point of HBr is higher than that of HCl.This is probably because HBr has
A)fewer electrons.
B)larger London dispersion interactions.
C)hydrogen bonding.
D)larger dipole-dipole interactions.
E)more ionic character.
A)fewer electrons.
B)larger London dispersion interactions.
C)hydrogen bonding.
D)larger dipole-dipole interactions.
E)more ionic character.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
24
Which compound below is capable of dipole-dipole interactions?
A)CF4
B)CS2
C)PCl3
D)SF6
E)BF3
A)CF4
B)CS2
C)PCl3
D)SF6
E)BF3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
25
Which statement below is FALSE?
A)When an ionic compound dissolves in water, the ions interact with the permanent dipoles of the water molecules.
B)Water molecules orient such that their oxygen atoms point toward an anion in solution.
C)Liquid ammonia, NH3, like water, could experience ion-dipole interactions with dissolved ions.
D)Water molecules in the inner sphere of hydration of an ion are involved in ion-dipole interactions.
E)Hydrated ions are stabilized by the surrounding water molecules.
A)When an ionic compound dissolves in water, the ions interact with the permanent dipoles of the water molecules.
B)Water molecules orient such that their oxygen atoms point toward an anion in solution.
C)Liquid ammonia, NH3, like water, could experience ion-dipole interactions with dissolved ions.
D)Water molecules in the inner sphere of hydration of an ion are involved in ion-dipole interactions.
E)Hydrated ions are stabilized by the surrounding water molecules.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
26
For a molecule to exhibit dipole-dipole interactions, it must
A)have a temporary dipole moment.
B)have a hydrogen bound to an oxygen, nitrogen, or fluorine.
C)have a permanent dipole moment.
D)be an ion.
E)have three or more atoms.
A)have a temporary dipole moment.
B)have a hydrogen bound to an oxygen, nitrogen, or fluorine.
C)have a permanent dipole moment.
D)be an ion.
E)have three or more atoms.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
27
Which pair of compounds below lists the molecule with the stronger dipole-dipole forces first?
A)CH2Br2 and CH2Cl2
B)C3H8 and CH3CN
C)CH3)3N and CH3)3P
D)CH3)2S and CH3)2O
E)CH3F and CHF3
A)CH2Br2 and CH2Cl2
B)C3H8 and CH3CN
C)CH3)3N and CH3)3P
D)CH3)2S and CH3)2O
E)CH3F and CHF3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
28
Which statement below regarding hydrogen bonding is FALSE?
A)Hydrogen bonds can form between like or unlike molecules.
B)Hydrogen bonds are intermediate in strength between covalent bonds and dipole-dipole forces.
C)Hydrogen bonding is irreversible.
D)Hydrogen must be directly bonded to F, N, or O to participate in hydrogen bonding.
E)Hydrogen bonding plays a key role in the shapes of molecules.
A)Hydrogen bonds can form between like or unlike molecules.
B)Hydrogen bonds are intermediate in strength between covalent bonds and dipole-dipole forces.
C)Hydrogen bonding is irreversible.
D)Hydrogen must be directly bonded to F, N, or O to participate in hydrogen bonding.
E)Hydrogen bonding plays a key role in the shapes of molecules.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
29
Which gaseous compound below is likely to condense at the highest temperature (gas liquid)?
A)NH3
B)NF3
C)CF4
D)PH3
E)PF3
A)NH3
B)NF3
C)CF4
D)PH3
E)PF3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
30
Dipole-dipole interactions typically are not as strong as ion-dipole interactions because
A)ion-dipole interactions involve partial charges caused by the equal sharing of electrons by atoms forming a bond.
B)dipole-dipole interactions involve the complete transfer of charge between atoms.
C)ion-dipole interactions involve only the partial transfer of charge between atoms.
D)ion-dipole interactions always involve water.
E)dipole-dipole interactions involve only partial charges caused by unequal sharing of electrons by atoms forming a bond.
A)ion-dipole interactions involve partial charges caused by the equal sharing of electrons by atoms forming a bond.
B)dipole-dipole interactions involve the complete transfer of charge between atoms.
C)ion-dipole interactions involve only the partial transfer of charge between atoms.
D)ion-dipole interactions always involve water.
E)dipole-dipole interactions involve only partial charges caused by unequal sharing of electrons by atoms forming a bond.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
31
Which compound below would you predict can experience only dispersion forces?
A)SF4
B)NO2
C)CCl4
D)H2CO
E)NF3
A)SF4
B)NO2
C)CCl4
D)H2CO
E)NF3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
32
A hydration sphere forms around an ion in aqueous solution due to
A)ion-dipole interactions.
B)ion-hydrogen-bonding interactions.
C)dispersion forces.
D)dipole-dipole interactions.
E)ion-induced dipole interactions.
A)ion-dipole interactions.
B)ion-hydrogen-bonding interactions.
C)dispersion forces.
D)dipole-dipole interactions.
E)ion-induced dipole interactions.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
33
When an ion is dissolved in water, which intermolecular forces are in effect: ion-dipole forces, dipole-dipole forces, and/or London dispersion forces?
A)London dispersion forces
B)dipole-dipole forces
C)ion-dipole forces
D)ion-dipole forces and London dispersion forces
E)ion-dipole forces, dipole-dipole forces, and London dispersion forces
A)London dispersion forces
B)dipole-dipole forces
C)ion-dipole forces
D)ion-dipole forces and London dispersion forces
E)ion-dipole forces, dipole-dipole forces, and London dispersion forces

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
34
You are interested in determining the solubility of ionic compounds in solvents other than water, and you decide to try liquid ammonia, NH3.Which statement below regarding your experiment is probably true?
A)The hydrogen ions in ammonia have partial negative charges and can stabilize Na- in solution.
B)The molecular dipole moment is too small to effectively interact with ions.
C)The hydrogen atoms in ammonia would orient toward both ions equally.
D)The lone pair of electrons on nitrogen in ammonia can act in a way similar to the lone pairs on oxygen in water.
E)Ion-dipole interactions exist only in water.
A)The hydrogen ions in ammonia have partial negative charges and can stabilize Na- in solution.
B)The molecular dipole moment is too small to effectively interact with ions.
C)The hydrogen atoms in ammonia would orient toward both ions equally.
D)The lone pair of electrons on nitrogen in ammonia can act in a way similar to the lone pairs on oxygen in water.
E)Ion-dipole interactions exist only in water.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
35
Based on their boiling points,which compound below will have the largest dipole-dipole interaction?
A)propane 231 K)
B)dimethyl ether 248 K)
C)acetonitrile 355 K)
D)methyl chloride 249 K)
E)butane 135 K)
A)propane 231 K)
B)dimethyl ether 248 K)
C)acetonitrile 355 K)
D)methyl chloride 249 K)
E)butane 135 K)

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
36
When sodium chloride dissolves in water, how do the water molecules orient around the ions?
A)Water molecules are randomly oriented around the ions.
B)The hydrogen atoms point toward both the sodium and the chloride ions.
C)The oxygen atoms point toward both the sodium ions and the chloride ions.
D)The hydrogen atoms point toward the sodium ions, and the oxygen atoms point toward the chloride ions.
E)The oxygen atoms point toward the sodium ions, and the hydrogen atoms point toward the chloride ions.
A)Water molecules are randomly oriented around the ions.
B)The hydrogen atoms point toward both the sodium and the chloride ions.
C)The oxygen atoms point toward both the sodium ions and the chloride ions.
D)The hydrogen atoms point toward the sodium ions, and the oxygen atoms point toward the chloride ions.
E)The oxygen atoms point toward the sodium ions, and the hydrogen atoms point toward the chloride ions.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
37
Which compound below is likely to have the highest boiling point?
A)CH3CF3
B)CH3CN
C)CH3)2CO
D)CH3CH2SH
E)CH3CH2OH
A)CH3CF3
B)CH3CN
C)CH3)2CO
D)CH3CH2SH
E)CH3CH2OH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
38
Which compound below will have the strongest dipole-dipole interactions between its molecules?
A)CF4
B)CO2
C)CH3F
D)H2CO
E)CH3Cl
A)CF4
B)CO2
C)CH3F
D)H2CO
E)CH3Cl

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
39
Which is the strongest interaction for substances with similar mass?
A)ion-dipole
B)hydrogen bonding
C)dipole-dipole
D)London dispersion
E)ion-induced dipole
A)ion-dipole
B)hydrogen bonding
C)dipole-dipole
D)London dispersion
E)ion-induced dipole

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
40
Which compound below is capable of hydrogen bonding with itself in the liquid state?
A)CHCl3
B)H2CO
C)HCN
D)BeH2
E)CH3OH
A)CHCl3
B)H2CO
C)HCN
D)BeH2
E)CH3OH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
41
Which pair of compounds below is most likely to be miscible?
A)CCl4 and Br2
B)Br2 and HCl
C)HF and CCl4
D)H2O and CH3CH2CH2CH3
E)CCl4 and NH3
A)CCl4 and Br2
B)Br2 and HCl
C)HF and CCl4
D)H2O and CH3CH2CH2CH3
E)CCl4 and NH3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
42
Stearic acid, a fatty acid commonly found in animal fat, has a condensed chemical formula of CH3(CH2)16COOH.It is a common component of soaps and detergents.Which stataement below is FALSE?
A)The -COOH group would experience hydrogen bonding with water.
B)The long alkane "tail," CH3(CH2)16-, is hydrophobic.
C)Stearic acid and water are probably miscible.
D)Stearic acid would probably dissolve in nonpolar solvents such as hexane, C6H14.
E)Stearic acid contains hydrophilic and hydrophobic regions.
A)The -COOH group would experience hydrogen bonding with water.
B)The long alkane "tail," CH3(CH2)16-, is hydrophobic.
C)Stearic acid and water are probably miscible.
D)Stearic acid would probably dissolve in nonpolar solvents such as hexane, C6H14.
E)Stearic acid contains hydrophilic and hydrophobic regions.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
43
Molecular nitrogen (N2) interacts with water and is sparingly soluble in water due to
A)dispersion forces.
B)dipole-induced dipole forces.
C)ion-dipole forces.
D)hydrogen bonding.
E)dipole-dipole forces.
A)dispersion forces.
B)dipole-induced dipole forces.
C)ion-dipole forces.
D)hydrogen bonding.
E)dipole-dipole forces.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
44
Which compound below would you expect to be most soluble in water?
A)CF4
B)CCl4
C)CH2Cl2
D)CH4
E)CH3OH
A)CF4
B)CCl4
C)CH2Cl2
D)CH4
E)CH3OH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
45
Suppose a solution that is 70% isopropyl alcohol and 30% water by mass has a density of 0.785 g/mL.Which statement below regarding this solution is true? Isopropyl alcohol has a condensed formula of CH3)2CHOH and a molar mass of 60.09 g/mol.
A)Isopropyl alcohol is the solvent.
B)Isopropyl alcohol can hydrogen bond with water and with itself.
C)100 mL of this solution would have a mass of 785 grams.
D)The number of moles of isopropyl alcohol is greater than the number of moles of water present.
E)The solution will separate over time because the intermolecular forces between water molecules are so strong.
A)Isopropyl alcohol is the solvent.
B)Isopropyl alcohol can hydrogen bond with water and with itself.
C)100 mL of this solution would have a mass of 785 grams.
D)The number of moles of isopropyl alcohol is greater than the number of moles of water present.
E)The solution will separate over time because the intermolecular forces between water molecules are so strong.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
46
Which choice below is not equal to a standard atmosphere (atm)?
A)1013 mb
B)105 Pa
C)101.3 kPa
D)1.013 * 105 N/m2
E)the average pressure at sea level
A)1013 mb
B)105 Pa
C)101.3 kPa
D)1.013 * 105 N/m2
E)the average pressure at sea level

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
47
Which alcohol should be most soluble in a nonpolar solvent such as hexane, C6H14?
A)CH3OH
B)CH3CH2OH
C)CH3CH2CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH
A)CH3OH
B)CH3CH2OH
C)CH3CH2CH2OH
D)CH3CH2CH2CH2OH
E)CH3CH2CH2CH2CH2OH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
48
A substance that is will be insoluble in water, but a substance that is will be soluble in water.
A)hydrophobic; immiscible
B)hydrophilic; hydrophobic
C)hydrophilic; miscible
D)hydrophobic; hydrophilic
E)miscible; immiscible
A)hydrophobic; immiscible
B)hydrophilic; hydrophobic
C)hydrophilic; miscible
D)hydrophobic; hydrophilic
E)miscible; immiscible

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
49
At the critical point,
A)all the liquid has evaporated.
B)the densities of the solid and liquid are the same.
C)the densities of the solid and the gas are the same.
D)the densities of the gas and the liquid are the same.
E)a critical mass has been reached.
A)all the liquid has evaporated.
B)the densities of the solid and liquid are the same.
C)the densities of the solid and the gas are the same.
D)the densities of the gas and the liquid are the same.
E)a critical mass has been reached.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
50
At the point marked with a dot on the phase diagram, the solid will 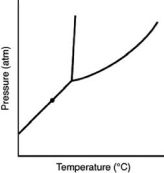
A)be indistinguishable from the gas.
B)boil.
C)melt.
D)sublime.
E)freeze.

A)be indistinguishable from the gas.
B)boil.
C)melt.
D)sublime.
E)freeze.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
51
Which statement below regarding the physical state of a substance is true?
A)All substances expand as they melt or boil.
B)The physical state observed is largely independent of attractive forces between the substance's constituent particles.
C)The physical state observed depends only on temperature.
D)The solid and liquid phases are unaffected by changes in pressure.
E)Changes in temperature cause changes in the energies of the substance's constituent particles.
A)All substances expand as they melt or boil.
B)The physical state observed is largely independent of attractive forces between the substance's constituent particles.
C)The physical state observed depends only on temperature.
D)The solid and liquid phases are unaffected by changes in pressure.
E)Changes in temperature cause changes in the energies of the substance's constituent particles.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
52
Which statement below is true?
A)Nonpolar molecules can dissolve in polar solvents.
B)Gases are soluble only in nonpolar solvents.
C)Ionic compounds cannot dissolve in any solvent other than water.
D)Polar molecules cannot dissolve in nonpolar solvents.
E)Solubility depends only slightly on the strength of solute-solute attractive forces.
A)Nonpolar molecules can dissolve in polar solvents.
B)Gases are soluble only in nonpolar solvents.
C)Ionic compounds cannot dissolve in any solvent other than water.
D)Polar molecules cannot dissolve in nonpolar solvents.
E)Solubility depends only slightly on the strength of solute-solute attractive forces.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
53
Which compound below would you most appropriately call hydrophobic?
A)CH4
B)H2CO
C)CO
D)HCl
E)NaCl
A)CH4
B)H2CO
C)CO
D)HCl
E)NaCl

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
54
Hydrophilic substances
A)are immiscible in water.
B)are insoluble in water.
C)are soluble in water.
D)are soluble in nonpolar solvents.
E)generally do not hydrogen bond.
A)are immiscible in water.
B)are insoluble in water.
C)are soluble in water.
D)are soluble in nonpolar solvents.
E)generally do not hydrogen bond.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
55
A phase diagram shows the states of a substance as a function of and .
A)pressure; volume
B)volume; temperature
C)pressure; temperature
D)concentration; temperature
E)density; pressure
A)pressure; volume
B)volume; temperature
C)pressure; temperature
D)concentration; temperature
E)density; pressure

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
56
Which compound below would be most soluble in benzene, C6H6?
A)H2O
B)H2COH)2
C)PCl3
D)CCl4
E)CH3CH2OH
A)H2O
B)H2COH)2
C)PCl3
D)CCl4
E)CH3CH2OH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
57
The solubility of a compound may depend on many factors, including
A)solute-solvent interactions.
B)solute-solute interactions.
C)solvent-solvent interactions.
D)physical parameters such as temperature.
E)all of the items listed.
A)solute-solvent interactions.
B)solute-solute interactions.
C)solvent-solvent interactions.
D)physical parameters such as temperature.
E)all of the items listed.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
58
You decide to experiment with carbon disulfide CS2) as a solvent.Which statement below is probably true? Carbon disulfide is a colorless liquid with a density of 1.261 g/mL and a molar mass of 76.14 g/mol.
A)CS2 is probably a good solvent for alkanes carbon-hydrogen molecules containing only single bonds).
B)Water is probably miscible with CS2.
C)For a substance to dissolve in CS2, dipole-dipole interactions between CS2 molecules would have to be broken.
D)Solutes that experience hydrogen bonding should dissolve readily in CS2.
E)Nonpolar gases such as N2 and O2 are probably completely insoluble in CS2.
A)CS2 is probably a good solvent for alkanes carbon-hydrogen molecules containing only single bonds).
B)Water is probably miscible with CS2.
C)For a substance to dissolve in CS2, dipole-dipole interactions between CS2 molecules would have to be broken.
D)Solutes that experience hydrogen bonding should dissolve readily in CS2.
E)Nonpolar gases such as N2 and O2 are probably completely insoluble in CS2.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
59
Which pair of compounds below is most likely to be immiscible?
A)Br2 and C6H6
B)H2O and CH3CH2OH
C)CCl4 and H2CO
D)CH3OH and CH3CH2OH
E)H2O and NH3
A)Br2 and C6H6
B)H2O and CH3CH2OH
C)CCl4 and H2CO
D)CH3OH and CH3CH2OH
E)H2O and NH3

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
60
At the triple point of a substance,
A)three phases are present in equilibrium.
B)the solid completely sublimes.
C)the gas completely condenses to a liquid.
D)only the solid and liquid are in equilibrium.
E)only one phase appears to be present.
A)three phases are present in equilibrium.
B)the solid completely sublimes.
C)the gas completely condenses to a liquid.
D)only the solid and liquid are in equilibrium.
E)only one phase appears to be present.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
61
Consider the phase diagram for a substance shown here.The solid phase is than the liquid phase. 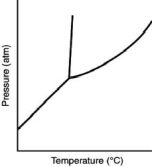
A)more dense
B)less dense
C)more massive
D)less massive
E)hotter

A)more dense
B)less dense
C)more massive
D)less massive
E)hotter

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
62
Which statement below best explains why the density of water decreases as it is cooled from 4.0 C to its normal freezing point?
A)Water molecules decrease in size as they cool because energy is released.
B)A regular, repeating network of hydrogen bonds between water molecules is formed.
C)Water molecules become more rigid as they cool.
D)Hydrogen bonds in liquid water are longer than they are in ice.
E)All substances contract just before freezing.
A)Water molecules decrease in size as they cool because energy is released.
B)A regular, repeating network of hydrogen bonds between water molecules is formed.
C)Water molecules become more rigid as they cool.
D)Hydrogen bonds in liquid water are longer than they are in ice.
E)All substances contract just before freezing.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
63
The temperature at point b in the phase diagram below is the _______
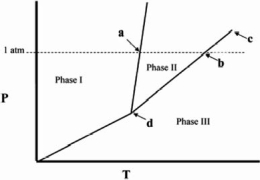
A)critical point.
B)triple point.
C)transition point.
D)normal freezing point.
E)normal boiling point.

A)critical point.
B)triple point.
C)transition point.
D)normal freezing point.
E)normal boiling point.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
64
Arrange the following molecules in order of increasing boiling point: CH2Cl2, CH3OH, CH3CH3, CH3F

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
65
Point c in the phase diagram below is the _______
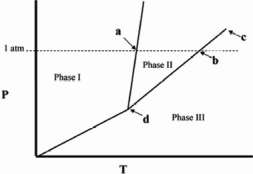
A)critical point.
B)triple point.
C)transition point.
D)normal freezing point.
E)normal boiling point.

A)critical point.
B)triple point.
C)transition point.
D)normal freezing point.
E)normal boiling point.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
66
Which statement below about the following phase diagram is FALSE? 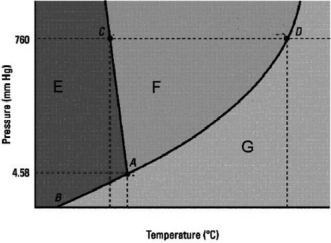
A)The solid phase is more dense than the liquid phase.
B)D is the normal boiling point.
C)The melting point decreases as pressure increases.
D)The normal melting point is at a lower temperature than the triple point.
E)E, F, and G label in that order the solid, liquid, and gas phases.

A)The solid phase is more dense than the liquid phase.
B)D is the normal boiling point.
C)The melting point decreases as pressure increases.
D)The normal melting point is at a lower temperature than the triple point.
E)E, F, and G label in that order the solid, liquid, and gas phases.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
67
Which statement below regarding capillary action is FALSE?
A)Capillary action refers to the rise of a liquid in a narrow tube.
B)The molecules can move in opposition to gravity.
C)The molecules in the liquid are attracted to each other.
D)An external force is required to cause the movement of the liquid.
E)The phenomenon is caused by both adhesive and cohesive forces.
A)Capillary action refers to the rise of a liquid in a narrow tube.
B)The molecules can move in opposition to gravity.
C)The molecules in the liquid are attracted to each other.
D)An external force is required to cause the movement of the liquid.
E)The phenomenon is caused by both adhesive and cohesive forces.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
68
Difluoromethane (CH2F2) has a dipole moment of 1.93 D and a boiling point of -52 C.Dichloromethane (CH2Cl2) has a dipole moment of 1.60 D and a boiling point of 40 C.Why is the boiling point of dichloromethane so much higher than that of difluoromethane?

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
69
Which liquid below will have the highest surface tension?
A)acetonitrile, CH3CN
B)acetone, CH3)2CO
C)water, H2O
D)ethanol, CH2CH2OH
E)ethylene glycol, HOCH2CH2OH
A)acetonitrile, CH3CN
B)acetone, CH3)2CO
C)water, H2O
D)ethanol, CH2CH2OH
E)ethylene glycol, HOCH2CH2OH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
70
The temperature at point a in the phase diagram below is the 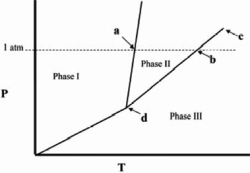
A)critical point.
B)triple point.
C)transition point.
D)normal freezing point.
E)normal boiling point.

A)critical point.
B)triple point.
C)transition point.
D)normal freezing point.
E)normal boiling point.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
71
Water forms a concave meniscus in a glass tube because
A)the cohesive forces between water molecules are larger than the adhesive forces between water molecules and the glass wall.
B)the adhesive forces between water molecules and the glass wall are larger than the cohesive forces between water molecules.
C)water molecules form hydrogen bonds with each other.
D)water molecules repel each other.
E)the glass wall is wet.
A)the cohesive forces between water molecules are larger than the adhesive forces between water molecules and the glass wall.
B)the adhesive forces between water molecules and the glass wall are larger than the cohesive forces between water molecules.
C)water molecules form hydrogen bonds with each other.
D)water molecules repel each other.
E)the glass wall is wet.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
72
Why does HI boil at a higher temperature than HBr even though the dipole moment of HBr 0.82 D) is larger than that of HI 0.38 D)?

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following ions would you predict experiences the strongest ion-dipole forces in water: Na+, K+, Mg2+, Ca2+, Br-, or I -? State your reasoning.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
74
The boiling points of group IVA hydrides increases in the order CH4 SiH4 GeH4 SnH4.Which molecule experiences the strongest attractive forces and why?

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
75
Point d in the phase diagram below is the _______
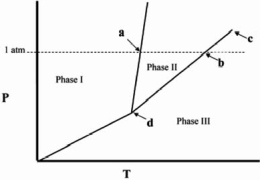
A)critical point.
B)triple point.
C)transition point.
D)normal freezing point.
E)normal boiling point.

A)critical point.
B)triple point.
C)transition point.
D)normal freezing point.
E)normal boiling point.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
76
Explain why the boiling point of nitrogen (N2, 77 K) is much lower than that of carbon monoxide (CO, 84 K) even though both have the same number of electrons and protons and nearly the same mass.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
77
What structural characteristics must a molecule have in order to hydrogen bond?

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
78
Arrange the following in order of increasing boiling point: Xe, Ar, Ne, F2, and Br2.Explain the reason for your ranking

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
79
Why do the strengths of dispersion interactions generally increase with the molar mass of the compound?

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
80
The resistance of a liquid to an increase in its surface area is
A)surface tension.
B)viscosity.
C)capillary action.
D)a meniscus.
E)density.
A)surface tension.
B)viscosity.
C)capillary action.
D)a meniscus.
E)density.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck


