Deck 10: Properties of Gases the Air We Breathe
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/164
Play
Full screen (f)
Deck 10: Properties of Gases the Air We Breathe
1
_____is how gases spread among each other; ____is how gases escape through a hole in a container.
A)Diffusion; effusion
B)Effusion; diffusion
C)Effusion; effusion
D)Diffusion; diffusion
A)Diffusion; effusion
B)Effusion; diffusion
C)Effusion; effusion
D)Diffusion; diffusion
Diffusion; effusion
2
Which set of gases is listed from highest to lowest root-mean-square speed at 25 C?
A)HCN Cl2 CO2 HBr
B)CO2 HCN Cl2 HBr
C)HBr Cl2 HCN CO2
D)HBr Cl2 CO2 HCN
E)HCN CO2 Cl2 HBr
A)HCN Cl2 CO2 HBr
B)CO2 HCN Cl2 HBr
C)HBr Cl2 HCN CO2
D)HBr Cl2 CO2 HCN
E)HCN CO2 Cl2 HBr
HCN CO2 Cl2 HBr
3
Which of the following statements regarding the kinetic molecular theory of gases is NOT correct?
A)The gas particles are assumed to occupy negligible volume.
B)At a given temperature, the gas particles have the same velocity.
C)The gas particles are in constant, rapid, random motion.
D)The average kinetic energy of the gas particles is directly proportional to the absolute temperature.
E)Energy can be transferred but not lost as gas particles collide with each other and the container walls.
A)The gas particles are assumed to occupy negligible volume.
B)At a given temperature, the gas particles have the same velocity.
C)The gas particles are in constant, rapid, random motion.
D)The average kinetic energy of the gas particles is directly proportional to the absolute temperature.
E)Energy can be transferred but not lost as gas particles collide with each other and the container walls.
At a given temperature, the gas particles have the same velocity.
4
Which of the following is NOT an assumption made in the kinetic molecular theory of gases?
A)The size of a molecule is negligible.
B)Gas molecules move constantly and randomly throughout the space they occupy.
C)The average kinetic energy of a gas molecule is directly proportional to the absolute temperature of the gas.
D)Collisions of gas molecules with each other and with the walls of the container are elastic.
E)Gas molecules attract and repel each other.
A)The size of a molecule is negligible.
B)Gas molecules move constantly and randomly throughout the space they occupy.
C)The average kinetic energy of a gas molecule is directly proportional to the absolute temperature of the gas.
D)Collisions of gas molecules with each other and with the walls of the container are elastic.
E)Gas molecules attract and repel each other.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements regarding how gases occupy container volumes is NOT correct?
A)Gases fill the entire volume of a container regardless of pressure.
B)The pressure exerted by a sample of gas in a container at a given temperature is uniform in all directions.
C)As the temperature decreases, the fraction of the container volume occupied by gas molecules decreases.
D)The volume unoccupied by gas molecules is spread uniformly throughout the container volume.
E)If gases confined to a smaller volume are allowed to enter a larger volume, they expand and distribute evenly throughout the new space available.
A)Gases fill the entire volume of a container regardless of pressure.
B)The pressure exerted by a sample of gas in a container at a given temperature is uniform in all directions.
C)As the temperature decreases, the fraction of the container volume occupied by gas molecules decreases.
D)The volume unoccupied by gas molecules is spread uniformly throughout the container volume.
E)If gases confined to a smaller volume are allowed to enter a larger volume, they expand and distribute evenly throughout the new space available.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
6
Which set of gases is listed from lowest to highest root-mean-square speed at 25 C?
A)Ne NO Ar CH4
B)Ar CH4 NO Ne
C)CH4 NO Ar Ne
D)Ar NO Ne CH4
E)CH4 Ne NO Ar
A)Ne NO Ar CH4
B)Ar CH4 NO Ne
C)CH4 NO Ar Ne
D)Ar NO Ne CH4
E)CH4 Ne NO Ar

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
7
The kinetic theory of gases assumes that collisions
A)with the walls of the container cause energy to be lost.
B)of molecules with each other cause energy to be lost.
C)are caused by molecules attracting each other.
D)do not result in a change in the energy of the molecules.
E)do not occur because the size of the molecules is negligible.
A)with the walls of the container cause energy to be lost.
B)of molecules with each other cause energy to be lost.
C)are caused by molecules attracting each other.
D)do not result in a change in the energy of the molecules.
E)do not occur because the size of the molecules is negligible.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
8
Which of these gases (Ar, N2O, H2) has the same average kinetic energy at 25 C as CO2?
A)Ar
B)N2O
C)H2
D)They are all the same as CO2.
E)They are all different from CO2.
A)Ar
B)N2O
C)H2
D)They are all the same as CO2.
E)They are all different from CO2.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements regarding ideal gases is NOT correct?
A)All gases are miscible with one another.
B)The pressure exerted by a gas increases as the amount of gas increases.
C)The rates at which gases travel are inversely proportional to their molar masses.
D)The fraction of a container occupied by the gas depends on its temperature.
E)The density of a gas is proportional to its molar mass at a given temperature and pressure.
A)All gases are miscible with one another.
B)The pressure exerted by a gas increases as the amount of gas increases.
C)The rates at which gases travel are inversely proportional to their molar masses.
D)The fraction of a container occupied by the gas depends on its temperature.
E)The density of a gas is proportional to its molar mass at a given temperature and pressure.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements regarding the density of gases is NOT correct?
A)The density of a gas is inversely proportional to its molar mass.
B)As the temperature goes up, the density of a gas does down.
C)The gas phase of a substance has a much lower density than the liquid or solid phase.
D)As volume increases, density decreases.
E)If the pressure on a gas increases, its density increases.
A)The density of a gas is inversely proportional to its molar mass.
B)As the temperature goes up, the density of a gas does down.
C)The gas phase of a substance has a much lower density than the liquid or solid phase.
D)As volume increases, density decreases.
E)If the pressure on a gas increases, its density increases.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
11
Assuming all of these samples of gas are at the same temperature, which will escape through a hole in a balloon at the lowest rate?
A)20 moles of H2 in a 12 L balloon
B)3 moles of Xe in an 18 L balloon
C)8 moles of Ne in a 24 L balloon
D)4 moles of Cl2 in a 16 L balloon
E)12 moles of He in a 24 L balloon
A)20 moles of H2 in a 12 L balloon
B)3 moles of Xe in an 18 L balloon
C)8 moles of Ne in a 24 L balloon
D)4 moles of Cl2 in a 16 L balloon
E)12 moles of He in a 24 L balloon

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
12
A gas is contained in the piston assembly and allowed to expand as in the following figure.Which point on the graph shows the correct final pressure and volume? 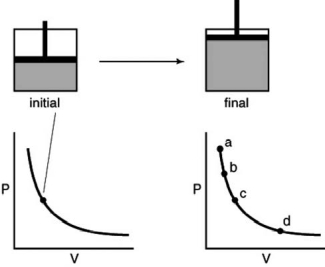
A)A
B)B
C)C
D)D
E)More information is needed.

A)A
B)B
C)C
D)D
E)More information is needed.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements regarding gas particle speed is NOT correct?
A)Gas particles have a distribution of speeds at a given temperature.
B)The largest number of gas particles travel at the most probable speed.
C)More massive gas particles have lower root-mean-square speeds than lighter particles at a given temperature.
D)Individual gas particles have different kinetic energies at a given temperature.
E)A particle traveling at the average speed has a kinetic energy equal to the average kinetic energy of all the gas particles.
A)Gas particles have a distribution of speeds at a given temperature.
B)The largest number of gas particles travel at the most probable speed.
C)More massive gas particles have lower root-mean-square speeds than lighter particles at a given temperature.
D)Individual gas particles have different kinetic energies at a given temperature.
E)A particle traveling at the average speed has a kinetic energy equal to the average kinetic energy of all the gas particles.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
14
The pressure of a gas is inversely proportional to
A)the volume of the gas.
B)the number of gas particles.
C)the temperature of the gas.
D)the mass of gas.
E)the density of the gas.
A)the volume of the gas.
B)the number of gas particles.
C)the temperature of the gas.
D)the mass of gas.
E)the density of the gas.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements regarding the mixing of gases is correct?
A)More massive gases settle to the bottom of a container, leaving lighter gases near the top.
B)The relative proportions of gases in a mixture vary throughout the container, depending on the pressure.
C)Nonpolar gases do not mix with polar gases.
D)All gas mixtures are homogeneous.
E)Gases mix more uniformly at lower temperatures.
A)More massive gases settle to the bottom of a container, leaving lighter gases near the top.
B)The relative proportions of gases in a mixture vary throughout the container, depending on the pressure.
C)Nonpolar gases do not mix with polar gases.
D)All gas mixtures are homogeneous.
E)Gases mix more uniformly at lower temperatures.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements regarding Graham's law of effusion is correct?
A)Effusion refers to the escape of a gas through a small hole into a region of lower pressure.
B)Gases at higher densities will effuse more rapidly.
C)If the molar mass doubles, the effusion rate doubles.
D)The effusion rate of helium is five times that of neon.
E)The effusion rate of n moles of argon in a 20 L container at 20 C is 10 times that observed with a 2 L container.
A)Effusion refers to the escape of a gas through a small hole into a region of lower pressure.
B)Gases at higher densities will effuse more rapidly.
C)If the molar mass doubles, the effusion rate doubles.
D)The effusion rate of helium is five times that of neon.
E)The effusion rate of n moles of argon in a 20 L container at 20 C is 10 times that observed with a 2 L container.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
17
To which of the following is the volume of a gas NOT directly proportional?
A)the temperature of the gas
B)the number of gas particles
C)the pressure of the gas
D)the average kinetic energy of the gas
E)the density of the gas
A)the temperature of the gas
B)the number of gas particles
C)the pressure of the gas
D)the average kinetic energy of the gas
E)the density of the gas

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
18
Which set of gases is listed from slowest to fastest effusion rate?
A)Xe SO2 CO2 SF4
B)CO2 SO2 SF4 Xe
C)Xe SF4 SO2 CO2
D)CO2 SF4 SO2 Xe
E)Xe SO2 SF4 CO2
A)Xe SO2 CO2 SF4
B)CO2 SO2 SF4 Xe
C)Xe SF4 SO2 CO2
D)CO2 SF4 SO2 Xe
E)Xe SO2 SF4 CO2

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following gases will escape through a hole in a balloon at the highest rate?
A)Kr
B)Ar
C)N2O
D)NO2
E)NO
A)Kr
B)Ar
C)N2O
D)NO2
E)NO

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
20
What is the average kinetic energy in (kJ/mol) of nitrous oxide (N2O) molecules at 30.0 C?
A)0.906 kJ/mol
B)3.78 kJ/mol
C)288 kJ/mol
D)147 kJ/mol
E)2.52 kJ/mol
A)0.906 kJ/mol
B)3.78 kJ/mol
C)288 kJ/mol
D)147 kJ/mol
E)2.52 kJ/mol

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
21
You need to hold the pressure of N2O gas in a balloon constant overnight, so you place it in a glove box that may contain a variety of gases.With which gas should you fill the glove box?
A)air ~78% N2 and 21% O2)
B)O2
C)Ar
D)N2
E)CO2
A)air ~78% N2 and 21% O2)
B)O2
C)Ar
D)N2
E)CO2

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
22
_____-is how gases escape through a hole in a container; _____is how gases spread among each other.
A)Diffusion; effusion
B)Effusion; diffusion
C)Effusion; effusion
D)Diffusion; diffusion
A)Diffusion; effusion
B)Effusion; diffusion
C)Effusion; effusion
D)Diffusion; diffusion

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
23
Which set of gases is listed from slowest to fastest diffusion rate?
A)HCN H2 He O3
B)H2 He HCN O3
C)O3 HCN He H2
D)HCN He O3 H2
E)H2 HCN He O3
A)HCN H2 He O3
B)H2 He HCN O3
C)O3 HCN He H2
D)HCN He O3 H2
E)H2 HCN He O3

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
24
About how long would it take hydrogen cyanide gas (HCN) to travel 10.0 meters at 25.0 C, when its average kinetic energy is 3.72 kJ/mol?
C, when its average kinetic energy is 3.72 kJ/mol?
(1 J = 1 kg . m2/s2; 1 mol = 6.02 * 1023)
A)36.3 microseconds
B)19.1 milliseconds
C)27.0 milliseconds
D)275 milliseconds
E)524 milliseconds
 C, when its average kinetic energy is 3.72 kJ/mol?
C, when its average kinetic energy is 3.72 kJ/mol? (1 J = 1 kg . m2/s2; 1 mol = 6.02 * 1023)
A)36.3 microseconds
B)19.1 milliseconds
C)27.0 milliseconds
D)275 milliseconds
E)524 milliseconds

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
25
The diffusion rate of helium in a lecture hall was found to be 25.0 m/s at 25 C.How long will it take poisonous phosgene gas, COCl2, to diffuse 10.0 m in the same room?
A)2.01 s
B)0.400 s
C)12.4 s
D)61.8 s
E)9.89 s
A)2.01 s
B)0.400 s
C)12.4 s
D)61.8 s
E)9.89 s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
26
What is the root-mean-square speed of HF at 37.5 C?
A)6.84 m/s
B)19.7 m/s
C)61.8 m/s
D)216 m/s
E)622 m/s
A)6.84 m/s
B)19.7 m/s
C)61.8 m/s
D)216 m/s
E)622 m/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
27
Determine the root-mean-square speed of methane (CH4) molecules that have an average kinetic energy of 10.5 J/mol.
A)337 m/s
B)1.14 m/s
C)2.81 * 1013 m/s
D)36.2 m/s
E)1150 m/s
A)337 m/s
B)1.14 m/s
C)2.81 * 1013 m/s
D)36.2 m/s
E)1150 m/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
28
At a given temperature, the effusion rate of nitrogen gas is times the rate of krypton.
A)2.99
B)0.409
C)2.45
D)0.578
E)1.73
A)2.99
B)0.409
C)2.45
D)0.578
E)1.73

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
29
Helium-neon HeNe) lasers contain a low-pressure mixture of He and Ne gases.How many times greater is the root-mean-square speed of He atoms than Ne atoms in a HeNe laser?
A)5.0
B)0.20
C)2.7
D)1.6
E)0.45
A)5.0
B)0.20
C)2.7
D)1.6
E)0.45

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate the root-mean-square speed of an O2 molecule with an average kinetic energy of 6.2 * 10-21 J at 25 C.
C.
(1 J = 1 kg . m2/s2; 1 mol = 6.02 * 1023)
A)20 pm/s
B)230 km/s
C)480 m/s
D)120 m/s
E)230 m/s
 C.
C.(1 J = 1 kg . m2/s2; 1 mol = 6.02 * 1023)
A)20 pm/s
B)230 km/s
C)480 m/s
D)120 m/s
E)230 m/s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
31
Which set of gases is listed from fastest to slowest diffusion rate?
A)N2 NH3 Ne OF2
B)NH3 Ne N2 OF2
C)OF2 N2 Ne NH3
D)N2 Ne OF2 NH3
E)NH3 N2 Ne OF2
A)N2 NH3 Ne OF2
B)NH3 Ne N2 OF2
C)OF2 N2 Ne NH3
D)N2 Ne OF2 NH3
E)NH3 N2 Ne OF2

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
32
Air is primarily nitrogen and oxygen, but noble gases such as argon and helium can also be present.The following graph shows the speed distributions for these different gases at the same temperature.Which curve might represent nitrogen? 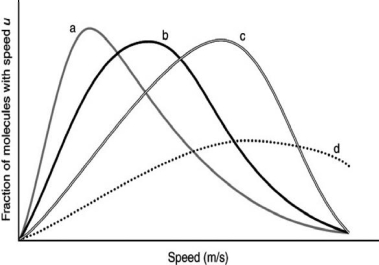
A)A
B)B
C)C
D)D
E)More information is needed.

A)A
B)B
C)C
D)D
E)More information is needed.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
33
At a given temperature, the rate at which bromine gas effuses is times the rate of helium gas.
A)0.224
B)0.158
C)6.32
D)4.47
E)0.0250
A)0.224
B)0.158
C)6.32
D)4.47
E)0.0250

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the average kinetic energy of an N2 molecule with a root-mean-square speed of 536 m/s.(1 J = 1 kg.m2/s2; 1 mol =6.02 *1023)
A)1.33 *10-20 J
B)6.68 * 10-21 J
C)1.25 * 10-23 J
D)2.49 *10-23 J
E)2.67 * 10-20 J
A)1.33 *10-20 J
B)6.68 * 10-21 J
C)1.25 * 10-23 J
D)2.49 *10-23 J
E)2.67 * 10-20 J

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the average kinetic energy of CO2 molecules with a root-mean-square speed of 629 m/s.Report your answer in kJ/mol.The mass of one CO2 molecule is 7.31 * 10-26 kg. (1 J = 1 kg . m2/s2; 1 mol =6.02 * 1023)
A)8700 kJ/mol
B)14 kJ/mol
C)17 kJ/mol
D)8.7 kJ/mol
E)28 kJ/mol
A)8700 kJ/mol
B)14 kJ/mol
C)17 kJ/mol
D)8.7 kJ/mol
E)28 kJ/mol

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is NOT a correct way to finish the following statement? In a sample of air at a given temperature,
A)the nitrogen and oxygen molecules have the same average speed.
B)the nitrogen and oxygen molecules have the same average kinetic energy.
C)some nitrogen molecules are moving slower than some oxygen molecules.
D)some nitrogen molecules are moving faster than some oxygen molecules.
E)all the molecules are moving.
A)the nitrogen and oxygen molecules have the same average speed.
B)the nitrogen and oxygen molecules have the same average kinetic energy.
C)some nitrogen molecules are moving slower than some oxygen molecules.
D)some nitrogen molecules are moving faster than some oxygen molecules.
E)all the molecules are moving.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
37
At a given temperature, oxygen gas effuses 1.489 times faster than which of the following gases?
A)CO2
B)SO2
C)Kr
D)Xe
E)Cl2
A)CO2
B)SO2
C)Kr
D)Xe
E)Cl2

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
38
The following graph shows the speed distributions for four different gases, all at the same temperature.Which of the curves is for the lightest gas? 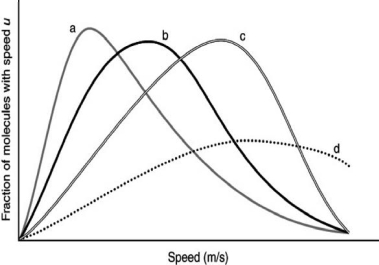
A)A
B)B
C)C
D)D
E)More information is needed.

A)A
B)B
C)C
D)D
E)More information is needed.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following will have the fastest effusion rate?
A)1.7 mol H2 in 5 L
B)0.2 mol Xe in 10 L
C)0.6 mol Ar in 15 L
D)2.5 mol He in 10 L
E)1.3 mol Ne in 20 L
A)1.7 mol H2 in 5 L
B)0.2 mol Xe in 10 L
C)0.6 mol Ar in 15 L
D)2.5 mol He in 10 L
E)1.3 mol Ne in 20 L

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
40
The root-mean-square speed of a gas is found to be 488.8 m/s at-14.3 C.What is the identity of the gas?
A)O2
B)CO2
C)Ar
D)HCN
E)CH4
A)O2
B)CO2
C)Ar
D)HCN
E)CH4

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
41
If the pressure in the eye of a hurricane is 892 mbar, what is the corresponding pressure in mmHg? Recall that 1.013 bar = 1 atm.
A)881 mmHg
B)678 mmHg
C)669 mmHg
D)904 mmHg
E)687 mmHg
A)881 mmHg
B)678 mmHg
C)669 mmHg
D)904 mmHg
E)687 mmHg

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
42
A barometer measures a pressure of 745 mmHg.What is this pressure in atm?
A)0.980 atm
B)1.02 atm
C)1.03 * 105 atm
D)1.00 * 104 atm
E)0.556 atm
A)0.980 atm
B)1.02 atm
C)1.03 * 105 atm
D)1.00 * 104 atm
E)0.556 atm

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
43
At the top of Mt.Everest, which has a height of 29,029 feet, atmospheric pressure is about 265 torr.Which of the following is NOT equivalent to 265 torr?
A)10.4 in Hg
B)35.3 kPa
C)5.12 psi
D)0.344 bar
E)0.349 atm
A)10.4 in Hg
B)35.3 kPa
C)5.12 psi
D)0.344 bar
E)0.349 atm

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
44
What height of water in meters) is necessary to measure a pressure of 760 mmHg? The density of mercury is 13.546 g/cm3, and the density of water is 1.00 g/cm3.
A)0.760 m
B)1.03 * 104 m
C)0.103 m
D)10.3 m
E)56.1 mm
A)0.760 m
B)1.03 * 104 m
C)0.103 m
D)10.3 m
E)56.1 mm

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
45
What force is exerted by a pressure of 2.153 atm on a mercury barometer with an inner diameter of 20.00 mm? Recall that the area of a circle is A = r2, 1 N = 1 kg m/s2, 1 Pa = 1 N/m2, and 1 atm = 1.013*105 Pa.
A)68.52 N
B)14.78 N
C)274.1 N
D)6.852 * 107 N
E)173.6 N
A)68.52 N
B)14.78 N
C)274.1 N
D)6.852 * 107 N
E)173.6 N

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
46
On a given day, the atmospheric pressure is reported to be 30.8 inches of mercury.Using 1 atm as normal atmospheric pressure, this day would be classified as
A)hot.
B)dense.
C)low pressure.
D)high pressure.
E)normal.
A)hot.
B)dense.
C)low pressure.
D)high pressure.
E)normal.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
47
The atmospheric pressure in the eye of a hurricane is found to be 26.6 inches of mercury, which is equivalent to
A)760 mmHg.
B)1.00* 105 Pa.
C)0.889 atm.
D)0.987 bar.
E)0.785 atm.
A)760 mmHg.
B)1.00* 105 Pa.
C)0.889 atm.
D)0.987 bar.
E)0.785 atm.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
48
Tungsten hexafluoride is one of the heaviest gases known, with a molar mass of 297.9 g/mol.At a given temperature, how long will it take WF6 to cover the distance H2 can travel in 25 seconds?
A)304 s
B)60.7 s
C)51.5 s
D)2.06 s
E)3690 s
A)304 s
B)60.7 s
C)51.5 s
D)2.06 s
E)3690 s

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
49
How many milliliters of mercury would exert a pressure of 235 kPa in a barometer with an inner diameter of 25.4 mm? The density of Hg is 13.546 g/mL.Recall that the area of a circle is A = r2, 1 N = 1 kg m/s2, 1 Pa = 1 N/m2, F = mg, and g = 9.81 m/s2.
A)164 mL
B)3580 mL
C)896 mL
D)119 mL
E)8.79 mL
A)164 mL
B)3580 mL
C)896 mL
D)119 mL
E)8.79 mL

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
50
Air, which is primarily nitrogen and oxygen, does contain a very small fraction of helium.What is the root-mean-square speed of helium at 25 C, where its average kinetic energy is about 3.72 kJ/mol? How many times faster or slower does it travel than N2? (1 J =1 kg .m2/s2; 1 mol =6.02 *1023)
A)1360 m/s; 2.65 times faster
B)515 m/s; 0.378 times slower
C)1860 km/s; 7.00 times faster
D)1360 km/s; 0.143 times slower
E)Both gases have the same urms at 25 C.
A)1360 m/s; 2.65 times faster
B)515 m/s; 0.378 times slower
C)1860 km/s; 7.00 times faster
D)1360 km/s; 0.143 times slower
E)Both gases have the same urms at 25 C.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
51
You wish to measure the changes in pressures of gases during the course of a reaction using a closed-end mercury manometer.Suppose 758 mmHg N2O4 is allowed to react to form NO2 according to the reaction N2O4 (g) 2 NO2 (g).The total pressure you measure as the reaction proceeds is the sum of the pressures of N2O4 and NO2 present.When the total pressure is 1039 mmHg, what is the pressure of N2O4?
A)477 mmHg
B)281 mmHg
C)576 mmHg
D)379 mmHg
E)553 mmHg
A)477 mmHg
B)281 mmHg
C)576 mmHg
D)379 mmHg
E)553 mmHg

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
52
What is the pressure in the gas bulb connected to the mercury manometer shown in the diagram if the ambient pressure is 756 torr? The heights labeled in the diagram are 15 mm and 21 mm. 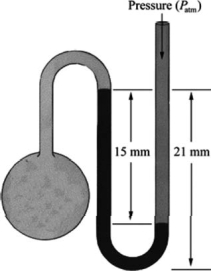
A)762 torr
B)778 torr
C)735 torr
D)771 torr
E)741 torr

A)762 torr
B)778 torr
C)735 torr
D)771 torr
E)741 torr

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
53
At a given temperature, an unknown gas takes 10.0 minutes to travel the distance helium gas travels in 1.66 minutes.What is the approximate molar mass of the unknown gas?
A)24.1 g/mol
B)68.9 g/mol
C)241 g/mol
D)36.3 g/mol
E)145 g/mol
A)24.1 g/mol
B)68.9 g/mol
C)241 g/mol
D)36.3 g/mol
E)145 g/mol

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following statements regarding instruments for measuring the pressures of gases is NOT correct?
A)A barometer is most often used to measure the pressures of samples of gas.
B)Open-end manometers are particularly useful for measuring gas pressures that are higher than atmospheric pressure.
C)Pressure sensors usually employ flexible diaphragms that distort based on changes in pressure.
D)The pressure of a gas connected to a closed-end mercury manometer is directly measured by the height of the mercury column observed.
E)The change in the height of the mercury column observed when a sample of gas is connected to an open-end manometer is directly related to the difference in the gas pressure and atmospheric pressure.
A)A barometer is most often used to measure the pressures of samples of gas.
B)Open-end manometers are particularly useful for measuring gas pressures that are higher than atmospheric pressure.
C)Pressure sensors usually employ flexible diaphragms that distort based on changes in pressure.
D)The pressure of a gas connected to a closed-end mercury manometer is directly measured by the height of the mercury column observed.
E)The change in the height of the mercury column observed when a sample of gas is connected to an open-end manometer is directly related to the difference in the gas pressure and atmospheric pressure.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
55
Two identical cement blocks are placed on a glass table in different positions, as shown in the diagram.Which statement about these blocks is correct? 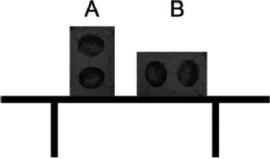
A)A exerts more pressure on the table.
B)B exerts more pressure on the table.
C)Both blocks exert the same pressure on the table.
D)A exerts more force on the table.
E)B exerts more force on the table.

A)A exerts more pressure on the table.
B)B exerts more pressure on the table.
C)Both blocks exert the same pressure on the table.
D)A exerts more force on the table.
E)B exerts more force on the table.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
56
What pressure will be measured (in Pa) if a gas is exerting a force of 10.00 N on a mercury barometer with an inner diameter of 10.00 mm? Recall that the area of a circle is A = r2, 1 N = 1 kg m/s2, and 1 Pa = 1 N/m2.
A)3.183 * 104 Pa
B)1.273 * 105 Pa
C)1.273 * 103 Pa
D)3.183 * 10-2 Pa
E)1.273 * 10-2 Pa
A)3.183 * 104 Pa
B)1.273 * 105 Pa
C)1.273 * 103 Pa
D)3.183 * 10-2 Pa
E)1.273 * 10-2 Pa

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
57
The cold inflation pressure of many car tires is 30.0 psi, which is the gauge pressure.If 1 atm = 14.7 psi, what is the gauge pressure of a car tire in torr?
A)2.04 torr
B)1.55* 103 torr
C)441 torr
D)0.580 torr
E)1.12 * 104 torr
A)2.04 torr
B)1.55* 103 torr
C)441 torr
D)0.580 torr
E)1.12 * 104 torr

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following substances will require the highest column for measuring an atmospheric pressure of 0.950 atm? Assume all the barometers have columns with the same diameter.)
A)mercury (d = 13.59 g/cm3)
B)ethanol (d = 0.785 g/cm3)
C)ethylene glycol (d = 1.09 g/cm3)
D)water (d = 1.00 g/cm3)
E)carbon tetrachloride (d = 1.58 g/cm3)
A)mercury (d = 13.59 g/cm3)
B)ethanol (d = 0.785 g/cm3)
C)ethylene glycol (d = 1.09 g/cm3)
D)water (d = 1.00 g/cm3)
E)carbon tetrachloride (d = 1.58 g/cm3)

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
59
What pressure (in Pa) will be exerted on the bottom of a 63.5 kg hiker's foot when she stands on a rusty nail? Assume the diameter of the tip of the nail is 1.00 mm.The acceleration due to gravity is 9.81 m/s2.Recall that the area of a circle is A = r2 and that 1 Pa =1 kg m-1 s-2.
A)1.98 * 102 Pa
B)7.93 * 102 Pa
C)1.98 * 108 Pa
D)7.93 * 108 Pa
E)7.93 *105 Pa
A)1.98 * 102 Pa
B)7.93 * 102 Pa
C)1.98 * 108 Pa
D)7.93 * 108 Pa
E)7.93 *105 Pa

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
60
The atmospheric pressure on Venus is approximately 9.3 MPa.What is this in atm? Recall that 1 bar =0.9869 atm, 1 atm = 101.325 kPa.
A)11 atm
B)9.4 atm
C)94 atm
D)92 atm
E)9.2 atm
A)11 atm
B)9.4 atm
C)94 atm
D)92 atm
E)9.2 atm

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
61
A sample of gas at -15.0 C is heated in a constant-volume container until the pressure is 65.0% higher than its original value.What is the final temperature of the gas?
A)153 C
B)426 C
C)202 C
D)124 C
E)298 C
A)153 C
B)426 C
C)202 C
D)124 C
E)298 C

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is NOT true?
A)P, V, T, and n for an ideal gas are state functions.
B)The ideal gas equation is PV = nRT, where R is the universal gas constant.
C)STP refers to 273 K and 1 bar, as defined by IUPAC.
D)The molar volume of a gas is the volume that one mole of an ideal gas occupies at STP.
E)The ratio PV/T has a constant value.
A)P, V, T, and n for an ideal gas are state functions.
B)The ideal gas equation is PV = nRT, where R is the universal gas constant.
C)STP refers to 273 K and 1 bar, as defined by IUPAC.
D)The molar volume of a gas is the volume that one mole of an ideal gas occupies at STP.
E)The ratio PV/T has a constant value.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
63
A sample of nitrogen gas has a volume of 10.0 L at 2.50 atm and 50.0 C.If the pressure is increased to 5.00 atm and the temperature decreases to 25.0 C, what volume does the gas occupy?
A)5.00 L
B)2.50 L
C)5.42 L
D)4.61 L
E)10.0 L
A)5.00 L
B)2.50 L
C)5.42 L
D)4.61 L
E)10.0 L

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
64
A sample of neon gas is contained in a 100.0 L cylinder with a movable piston at 25 C and 0.55 atm.If the absolute temperature doubles and the gas expands at constant pressure, approximately how many joules of P-V work does the gas do?
A)(-1300 J)
B)(-420 J)
C)(-5600 J)
D)(-2500 J)
E)(-940 J)
A)(-1300 J)
B)(-420 J)
C)(-5600 J)
D)(-2500 J)
E)(-940 J)

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
65
A sample of gas at 875 C is allowed to cool at constant pressure until its volume is one-fourth of its original value.What is the final temperature of the gas?
A)(-54.3 C)
B)13.9 C
C)219 C
D)287 C
E)383 C
A)(-54.3 C)
B)13.9 C
C)219 C
D)287 C
E)383 C

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
66
In an experiment, 25.0 mL of a gas with a pressure of 1.00 atm is contained in a balloon at 25.00 C.The balloon's temperature is adjusted until the pressure is 0.750 atm at a volume of 31.1 mL.What is the final temperature of the gas under the new conditions?
A)23.3 C
B)(-93.4 C)
C)(-46.5 C)
D)5.02 C
E)46.4 C
A)23.3 C
B)(-93.4 C)
C)(-46.5 C)
D)5.02 C
E)46.4 C

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
67
A weather balloon is inflated to 40.0 L and 1.00 atm at 21.3 C.It rises to an altitude where the pressure is 0.280 atm and the temperature is -48.6 C.What is the final volume of the balloon?
A)109 L
B)187 L
C)143 L
D)30.5 L
E)62.6 L
A)109 L
B)187 L
C)143 L
D)30.5 L
E)62.6 L

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following statements regarding temperature and absolute zero (-273.15 C) is NOT correct?
A)At constant P and n, the volume of a gas decreases as temperature decreases because the average kinetic energy of the particles decreases.
B)When a sample of gas is at any temperature above absolute zero, it implies that the gas has done pressure-volume work as long as there was an opposing pressure).
C)The concept of zero volume of a gas at absolute zero requires that the gas does not condense to form the liquid phase.
D)Equal moles of different gases occupy different volumes at constant pressure, but their extrapolated volume at 0 K appears to be zero.
E)Absolute zero has been reached experimentally by decreasing the volume of a gas to 1/273.15 of its original value.
A)At constant P and n, the volume of a gas decreases as temperature decreases because the average kinetic energy of the particles decreases.
B)When a sample of gas is at any temperature above absolute zero, it implies that the gas has done pressure-volume work as long as there was an opposing pressure).
C)The concept of zero volume of a gas at absolute zero requires that the gas does not condense to form the liquid phase.
D)Equal moles of different gases occupy different volumes at constant pressure, but their extrapolated volume at 0 K appears to be zero.
E)Absolute zero has been reached experimentally by decreasing the volume of a gas to 1/273.15 of its original value.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
69
A balloon is filled with 3.00 L of helium at a pressure of 765 torr.What is the volume of the balloon at an altitude where the pressure is 530 torr if the temperature remains constant?
A)0.231 L
B)4.33 L
C)2.08 L
D)1.00 L
E)3.00 L
A)0.231 L
B)4.33 L
C)2.08 L
D)1.00 L
E)3.00 L

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
70
Oxygen gas is formed by the decomposition of potassium chlorate at high temperatures according to the reaction 2 KClO3 (s) 2 KCl(s)+3 O2 (g).Suppose 1.23 g KClO3 is placed in a container connected to an open-end mercury manometer on a day when atmospheric pressure is 1.00 atm.Once the reaction is complete, the height of the mercury column in the U-tube on the side of the reaction container rises by 172 mmHg.What is the pressure of O2 gas produced by the reaction?
A)1348 torr
B)0.774 atm
C)22.9 kPa
D)0.559 bar
E)36.7 in Hg
A)1348 torr
B)0.774 atm
C)22.9 kPa
D)0.559 bar
E)36.7 in Hg

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
71
A gas has a volume of 50.0 mL at 3.50 atm.What is the volume at 372 mmHg if the temperature remains constant?
A)0.470 mL
B)5.31 L
C)0.358 L
D)6.99 mL
E)133 mL
A)0.470 mL
B)5.31 L
C)0.358 L
D)6.99 mL
E)133 mL

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
72
What is the molar volume of an ideal gas at 1.00 bar and 0.00 C?
A)22.4 L
B)22.7 L
C)22.1 L
D)15.0 L
E)27.2 L
A)22.4 L
B)22.7 L
C)22.1 L
D)15.0 L
E)27.2 L

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following statements regarding the gas laws is NOT correct?
A)As temperature increases at constant pressure, the volume increases so that the total force per area exerted by the gas particles on the container walls remains constant.
B)As temperature increases at constant volume, the frequency and energy of the collisions between the particles and the container walls increase.
C)The average number of collisions experienced by the particles can remain constant if the volume of the container can increase as the amount of gas increases at constant temperature and pressure.
D)As temperature increases at constant volume, more particles with higher average kinetic energy collide with each other and the container walls.
E)As particle density increases at constant temperature, the average energy of the collisions between the particles and the container walls decreases.
A)As temperature increases at constant pressure, the volume increases so that the total force per area exerted by the gas particles on the container walls remains constant.
B)As temperature increases at constant volume, the frequency and energy of the collisions between the particles and the container walls increase.
C)The average number of collisions experienced by the particles can remain constant if the volume of the container can increase as the amount of gas increases at constant temperature and pressure.
D)As temperature increases at constant volume, more particles with higher average kinetic energy collide with each other and the container walls.
E)As particle density increases at constant temperature, the average energy of the collisions between the particles and the container walls decreases.

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following is unimportant when using the ideal gas law?
A)the chemical identity of the gas sample
B)the temperature of the gas sample
C)the pressure of the gas sample
D)the volume of the container holding the gas sample
E)the amount of gas
A)the chemical identity of the gas sample
B)the temperature of the gas sample
C)the pressure of the gas sample
D)the volume of the container holding the gas sample
E)the amount of gas

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
75
A sample of gas at 475.0 C is allowed to cool at constant volume until its pressure is one-fifth of its original value.What is the final temperature of the gas?
A)(-50.67 C)
B)95.00 C
C)273.2 C
D)149.6 C
E)(-123.5 C)
A)(-50.67 C)
B)95.00 C
C)273.2 C
D)149.6 C
E)(-123.5 C)

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
76
A sample of oxygen gas at 25.0 C has its pressure increased 85.0% while its volume is decreased 35.0%.What is the final temperature of the gas?
A)(-80.1 C)
B)85.4 C
C)7.44 C
D)30.1 C
E)575 C
A)(-80.1 C)
B)85.4 C
C)7.44 C
D)30.1 C
E)575 C

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
77
You wish to measure the extent of reaction by monitoring pressure changes.Suppose 1.000 atm of tungsten hexafluoride gas at 400.00 C in a 20.0 L deposition chamber partially decomposes to form tungsten metal and fluorine gas.The products of the reaction at this point are cooled to 25.00 C.The resulting pressure, which is due to the sum of the pressures of F2 and leftover WF6, is 1.028 atm.What is the pressure due to the unreacted WF6?
WF6 (g) W(s)+ 3 F2 (g)
A)0.3395 atm
B)0.6605 atm
C)0.9830 atm
D)0.1230 atm
E)0.1504 atm
WF6 (g) W(s)+ 3 F2 (g)
A)0.3395 atm
B)0.6605 atm
C)0.9830 atm
D)0.1230 atm
E)0.1504 atm

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
78
A sample of argon gas at 2.90 atm and 35.0 mL is heated from 20.0 C to 50.0 C.If the pressure remains constant, what is the final volume of the gas?
A)38.6 mL
B)87.5 mL
C)31.8 mL
D)35.0 mL
E)52.5 mL
A)38.6 mL
B)87.5 mL
C)31.8 mL
D)35.0 mL
E)52.5 mL

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
79
A sample of neon gas at 745 torr and 60.0 L is compressed to 14.5 L.If the temperature remains constant, what is the final pressure of the gas?
A)1.17 torr
B)180 torr
C)3080 torr
D)761 torr
E)792 torr
A)1.17 torr
B)180 torr
C)3080 torr
D)761 torr
E)792 torr

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck
80
A sample of helium has a volume of 2.275 L at 10.25 atm and 30.0 C.If the volume is increased to 20.39 L and the temperature decreases to -20.0 C, what pressure does the gas exert?
A)1.37 atm
B)0.730 atm
C)0.136 atm
D)(-0.762 atm)
E)0.955 atm
A)1.37 atm
B)0.730 atm
C)0.136 atm
D)(-0.762 atm)
E)0.955 atm

Unlock Deck
Unlock for access to all 164 flashcards in this deck.
Unlock Deck
k this deck


