Deck 14: Chemical Equilibrium Equal but Opposite Reaction Rates
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/119
Play
Full screen (f)
Deck 14: Chemical Equilibrium Equal but Opposite Reaction Rates
1
Which statement below regarding the equilibrium constant is FALSE?
A)When K 1, the concentrations of products are much greater than the concentrations of reactants at equilibrium.
B)When K 1, the concentrations of reactants are much greater than the concentrations of products at equilibrium.
C)When K = 1, the concentrations of products and reactants at equilibrium are equal.
D)When K = 1, the forward and reverse rate constants are equal.
E)When K 1, the products and reactants come to equilibrium rapidly.
A)When K 1, the concentrations of products are much greater than the concentrations of reactants at equilibrium.
B)When K 1, the concentrations of reactants are much greater than the concentrations of products at equilibrium.
C)When K = 1, the concentrations of products and reactants at equilibrium are equal.
D)When K = 1, the forward and reverse rate constants are equal.
E)When K 1, the products and reactants come to equilibrium rapidly.
When K 1, the products and reactants come to equilibrium rapidly.
2
For the equilibrium 2HI(g) ⇄ H2 (g) + I2 (g), the equilibrium constant at 700 K is approximately 1.75 * 10-2 and the rate constant of the forward reaction is approximately 1.16*10-3 M -1 s-1.What is the value of the reverse rate constant?
A)6.63 *10-2 M -1 s-1
B)1.51* 101 M -1 s-1
C)1.16 *10-3 M -1 s-1
D)2.03 * 10-5 M -1 s-1
E)1.00 M -1 s-1
A)6.63 *10-2 M -1 s-1
B)1.51* 101 M -1 s-1
C)1.16 *10-3 M -1 s-1
D)2.03 * 10-5 M -1 s-1
E)1.00 M -1 s-1
6.63 *10-2 M -1 s-1
3
Which statement below regarding the law of mass action and the mass action expression is FALSE?
A)The law of mass action defines the equilibrium constant to be the ratio of the forward and reverse rate constants for a particular equilibrium expression.
B)The law of mass action states that the ratio of product concentrations to reactant concentrations, raised to powers equal to their coefficients in a balanced chemical reaction, has a characteristic value at a given temperature.
C)The mass action expression is equivalent to the equilibrium expression in form but may be applied to reaction mixtures that are not at equilibrium.
D)At a given temperature, the characteristic ratio of products to reactants at equilibrium will be the same regardless of the composition of the starting mixture.
E)At a given temperature, the equilibrium concentrations of products and reactants depend on the composition of the starting mixture.
A)The law of mass action defines the equilibrium constant to be the ratio of the forward and reverse rate constants for a particular equilibrium expression.
B)The law of mass action states that the ratio of product concentrations to reactant concentrations, raised to powers equal to their coefficients in a balanced chemical reaction, has a characteristic value at a given temperature.
C)The mass action expression is equivalent to the equilibrium expression in form but may be applied to reaction mixtures that are not at equilibrium.
D)At a given temperature, the characteristic ratio of products to reactants at equilibrium will be the same regardless of the composition of the starting mixture.
E)At a given temperature, the equilibrium concentrations of products and reactants depend on the composition of the starting mixture.
The law of mass action defines the equilibrium constant to be the ratio of the forward and reverse rate constants for a particular equilibrium expression.
4
For the equilibrium NO2 (g) +O2 (g) ⇄ NO(g) +O3 (g), the equilibrium constant at 300.0 K is approximately 2.9*10-35 and the rate constant of the reverse reaction is approximately 8.5 *106 M -1 s-1.What is the value of the forward rate constant?
A)2.1 * 10-21 M -1 s-1
B)3.4 *10-42 M -1 s-1
C)2.5 * 10-28 M -1 s-1
D)4.1 *1027 M -1 s-1
E)1.0 M -1 s-1
A)2.1 * 10-21 M -1 s-1
B)3.4 *10-42 M -1 s-1
C)2.5 * 10-28 M -1 s-1
D)4.1 *1027 M -1 s-1
E)1.0 M -1 s-1

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
5
A chemical equilibrium may be established by starting a reaction with
A)reactants only.
B)products only.
C)equal quantities of reactants and products.
D)any quantities of reactants and products.
E)all of the above
A)reactants only.
B)products only.
C)equal quantities of reactants and products.
D)any quantities of reactants and products.
E)all of the above

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
6
The equilibrium constant for the acid ionization of mercaptoethanol (HSCH2CH2OH ) is 1.91 *10-10.HSCH2CH2OH(aq) ⇄ H+ (aq) +SCH2CH2OH-(aq).Which statements) regarding this equilibrium is/are true? I.The reaction is product favored.
II.The reaction is reactant favored.
III.Equilibrium lies far to the right.
IV.Equilibrium lies far to the left.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)None are true, as the concentrations of reactants and products are comparable.
II.The reaction is reactant favored.
III.Equilibrium lies far to the right.
IV.Equilibrium lies far to the left.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)None are true, as the concentrations of reactants and products are comparable.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
7
Consider the equilibrium A +B ⇄ C.What is significant about the equilibrium state in which [B] = [C]?
A)[A]= K
B)[A] =[B] = [C]
C)[B] = [C] = K
D)[A] = 1/K
E)[C]/[B] = K
A)[A]= K
B)[A] =[B] = [C]
C)[B] = [C] = K
D)[A] = 1/K
E)[C]/[B] = K

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
8
The forward rate constant, kf, and reverse rate constant, kr, for a chemical reaction are not equal.Which statement below must be true?
A)The reaction will be unable to achieve equilibrium.
B)kf and kr will become equal as equilibrium is approached owing to concentration changes.
C)kf and kr will become equal as equilibrium is approached owing to temperature changes.
D)kf and kr will remain unequal, but the rates will become equal owing to concentration changes.
E)kf and kr will remain unequal, but the rates will become equal owing to temperature changes.
A)The reaction will be unable to achieve equilibrium.
B)kf and kr will become equal as equilibrium is approached owing to concentration changes.
C)kf and kr will become equal as equilibrium is approached owing to temperature changes.
D)kf and kr will remain unequal, but the rates will become equal owing to concentration changes.
E)kf and kr will remain unequal, but the rates will become equal owing to temperature changes.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
9
The equilibrium constant for the formation of hydrogen iodide from hydrogen and iodine-H2(g) + I2(s) ⇄ 2 HI(g)-is 45.0 at a certain temperature.Which statement(s) regarding this equilibrium is/are true? I.The reaction is product favored.
II.The reaction is reactant favored.
III.Equilibrium lies to the right.
IV.Equilibrium lies to the left.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)None are true, as the concentrations of reactants and products are essentially the same.
II.The reaction is reactant favored.
III.Equilibrium lies to the right.
IV.Equilibrium lies to the left.
A)I and III
B)I and IV
C)II and III
D)II and IV
E)None are true, as the concentrations of reactants and products are essentially the same.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
10
Which statement below concerning a chemical reaction at equilibrium is true?
A)Only the forward reaction stops.
B)Only the reverse reaction stops.
C)Both the forward and reverse reactions stop.
D)The rate constants for the forward and reverse reactions are equal.
E)The rates of the forward and reverse reactions are equal.
A)Only the forward reaction stops.
B)Only the reverse reaction stops.
C)Both the forward and reverse reactions stop.
D)The rate constants for the forward and reverse reactions are equal.
E)The rates of the forward and reverse reactions are equal.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
11
Which statement below regarding the equilibrium constant and its units is true?
A)K is unitless because the concentrations or partial pressures in the expression are theoretically ratios of concentration or partial pressure to an ideal value of 1.000 M or 1.000 atm.
B)K is unitless because the units of the concentrations or partial pressures in the expression are dropped for convenience.
C)K is unitless because the units of the concentrations or partial pressures in the expression cancel if the equilibrium expression is written correctly.
D)K has units that reflect the units of the concentrations or partial pressures in the equilibrium expression.
E)K has units of M or atm, depending on whether solutions or gases are involved.
A)K is unitless because the concentrations or partial pressures in the expression are theoretically ratios of concentration or partial pressure to an ideal value of 1.000 M or 1.000 atm.
B)K is unitless because the units of the concentrations or partial pressures in the expression are dropped for convenience.
C)K is unitless because the units of the concentrations or partial pressures in the expression cancel if the equilibrium expression is written correctly.
D)K has units that reflect the units of the concentrations or partial pressures in the equilibrium expression.
E)K has units of M or atm, depending on whether solutions or gases are involved.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
12
For an equilibrium reaction with K = 1.2 * 108, the forward rate constant was found to be 3.5*105.What is the value of the reverse rate constant?
A)3.5 *105
B)3.4*102
C)4.2 * 1013
D)6.0 * 10-
E)2.9 * 10-3
A)3.5 *105
B)3.4*102
C)4.2 * 1013
D)6.0 * 10-
E)2.9 * 10-3

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the equilibrium that exists between Fe2+ and Fe3+ ions in solution.The extra electron originally on one Fe2+ ion can be transferred to an Fe3+ ion.This amounts to no net reaction because iron(II) becomes iron(III) and iron(III) becomes iron(II).Which below about this equilibrium is true?
A)There is no activation energy for this reaction.
B)K = 1.
C)K =0.
D)There is no net reaction, so there is no value for K.
E)The concentrations of iron(II) and iron(III) change because of the electron transfer.
A)There is no activation energy for this reaction.
B)K = 1.
C)K =0.
D)There is no net reaction, so there is no value for K.
E)The concentrations of iron(II) and iron(III) change because of the electron transfer.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is/are equal once equilibrium is established?
A)the concentrations of reactant and products
B)the rates of the forward and reverse reactions
C)the time that a particular atom or molecule spends as a reactant and product
D)the rate constants of the forward and reverse reactions
E)All of the above are equal.
A)the concentrations of reactant and products
B)the rates of the forward and reverse reactions
C)the time that a particular atom or molecule spends as a reactant and product
D)the rate constants of the forward and reverse reactions
E)All of the above are equal.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement below regarding the concentration of products for a chemical reaction at equilibrium is true?
A)They will not change because there are no more reactants.
B)They will not change because the limiting reagent is gone.
C)They will not change because this is a constant for each reaction.
D)They will not change because the forward and reverse rates are equal.
E)They will change continually because of reversibility.
A)They will not change because there are no more reactants.
B)They will not change because the limiting reagent is gone.
C)They will not change because this is a constant for each reaction.
D)They will not change because the forward and reverse rates are equal.
E)They will change continually because of reversibility.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
16
A chemical equilibrium A ⇄ 2 B has a forward rate constant, kf =20.0 s-1, and a reverse rate constant, kr = 0.400 M -1 s-1.What is the value of the equilibrium constant for this system?
A)8.00
B)50.0
C)125
D)0.0200
E)25.0
A)8.00
B)50.0
C)125
D)0.0200
E)25.0

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
17
A chemical equilibrium A ⇄ 2 B has a forward rate constant, kf = 20.0 s-1, and a reverse rate constant, kr =0.400 M -1 s-1.If the system has a concentration of [A] = 0.10 M at equilibrium, what is the concentration of B at equilibrium?
A)0.0447
B)5.00
C)0.0224
D)2.24
E)22.4
A)0.0447
B)5.00
C)0.0224
D)2.24
E)22.4

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement below regarding reaction rates and equilibrium is FALSE?
A)For a reaction to attain equilibrium, reactants and products must remain in contact with each other.
B)The rate of the forward reaction is at its maximum when the reactants are first mixed.
C)The rate of the reverse reaction is at its maximum when the reactants are first mixed.
D)The rate of the forward reaction decreases as the system approaches equilibrium.
E)The rate of the reverse reaction increases as the system approaches equilibrium.
A)For a reaction to attain equilibrium, reactants and products must remain in contact with each other.
B)The rate of the forward reaction is at its maximum when the reactants are first mixed.
C)The rate of the reverse reaction is at its maximum when the reactants are first mixed.
D)The rate of the forward reaction decreases as the system approaches equilibrium.
E)The rate of the reverse reaction increases as the system approaches equilibrium.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the equilibrium 2 NOCl(g) ⇄ NO(g) + CL2 (g).At 800.0 K, the equilibrium constant is approximately 6.1 * 10-8.Which statement below about this equilibrium is true?
A)There is no activation energy for this reaction.
B)There is no net reaction, so there is no value for K.
C)K = 1.
D)kf is less than kr at equilibrium.
E)The forward reaction rate is less than the reverse reaction rate.
A)There is no activation energy for this reaction.
B)There is no net reaction, so there is no value for K.
C)K = 1.
D)kf is less than kr at equilibrium.
E)The forward reaction rate is less than the reverse reaction rate.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
20
For the equilibrium CO2 (g) +N2 (g) ⇄ CO(g) +N2O(g), the forward and reverse rate constants at 1200 K are 9.1 *10-11 M -1 s-1 and 1.5 *105 M -1 s-1, respectively.What is the value of the equilibrium constant for CO(g) + N2O(g) ⇄ CO2 (g) +N2 (g)?
A)1.6 *1015
B)1.4 *10-5
C)6.1 *10-16
D)7.1 * 104
E)1.0
A)1.6 *1015
B)1.4 *10-5
C)6.1 *10-16
D)7.1 * 104
E)1.0

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
21
The equilibrium constants for the two reactions are known:
M m++ 4 L- ⇄ [ML4]m-4 Kf
HL ⇄ H+ +L- Ka
What is the equilibrium constant, Koverall, for the reaction, M m++ 4 HL ⇄ [ML4 ]m + 4 +4H+?
A)KfKa
B)Kf+ 4Ka
C)Kf + Ka4
D)KfKa4
E)KfKa1/4
M m++ 4 L- ⇄ [ML4]m-4 Kf
HL ⇄ H+ +L- Ka
What is the equilibrium constant, Koverall, for the reaction, M m++ 4 HL ⇄ [ML4 ]m + 4 +4H+?
A)KfKa
B)Kf+ 4Ka
C)Kf + Ka4
D)KfKa4
E)KfKa1/4

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
22
For the chemical equilibrium aA + bB ⇄ cC, the value of the equilibrium constant K is 10.What is the value of the equilibrium constant for the reaction cC ⇄ aA + bB?
A)0.1
B)10
C)1
D)100
E)(-10)
A)0.1
B)10
C)1
D)100
E)(-10)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
23
Consider the following equilibrium: CO(g) +3 H2 (g) ⇄ CH4 (g) +H2O(g).If Kp = 1.61 *10-5 at 1400.0 K, calculate Kc.
A)1.40 * 10-7
B)1.85 * 10-3
C)541
D)0.212
E)1.22 * 10-9
A)1.40 * 10-7
B)1.85 * 10-3
C)541
D)0.212
E)1.22 * 10-9

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the following equilibrium: CH4 (g) +2 H2 S(g) ⇆ CS2 (g) +4 H2 (g).If Kp= 33.0 at 1400.0 K, calculate Kc.
A)3.79 * 103
B)2.50 *10-3
C)0.287
D)4.36 *105
E)3.48
A)3.79 * 103
B)2.50 *10-3
C)0.287
D)4.36 *105
E)3.48

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
25
The following reactions at 1000.0 K have the following Kp values: H2 (g) + CL2 (g) ⇄ 2 HCl(g) Kp = 3.8 * 104
H2 (g) + Br2 (g) ⇄ 2 HBr(g)) Kp =5.1 *108
Br2 (g) + CL2 (g) ⇄ 2 Br Cl(g) Kp = 0.20
What is Kp for the reaction HCl(g) + HBr(g) ⇄ BrCl(g) + H2(g) at 1000.0 K?
A)5.1 *10-7
B)1.0 *10-7
C)5.2*10-15
D)0.45
E)1.0 *10-14
H2 (g) + Br2 (g) ⇄ 2 HBr(g)) Kp =5.1 *108
Br2 (g) + CL2 (g) ⇄ 2 Br Cl(g) Kp = 0.20
What is Kp for the reaction HCl(g) + HBr(g) ⇄ BrCl(g) + H2(g) at 1000.0 K?
A)5.1 *10-7
B)1.0 *10-7
C)5.2*10-15
D)0.45
E)1.0 *10-14

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
26
Under what conditions are the values of Kc and Kp for a given gas-phase equilibrium the same?
A)There is no change in the moles of gas in the reaction.
B)There is no change in the temperature during the reaction.
C)The coefficients of the reactants and products are the same.
D)The pressure remains constant.
E)Either Kc or Kp = 1.
A)There is no change in the moles of gas in the reaction.
B)There is no change in the temperature during the reaction.
C)The coefficients of the reactants and products are the same.
D)The pressure remains constant.
E)Either Kc or Kp = 1.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
27
Consider the equilibrium 2 NOClg) CL2 g) + 2 NOg).When 2.000 atm NOCl is placed in an empty tank at 500.0 K and allowed to come to equilibrium, the equilibrium partial pressure of Cl2 is 0.226 atm.Calculate Kp.
A)0.0115
B)15.2
C)0.0193
D)0.0660
E)52.0
A)0.0115
B)15.2
C)0.0193
D)0.0660
E)52.0

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the following equilibrium: ClF3 (g) ⇄ ClF(g) + F2 (g).When 0.500 atm ClF3 is placed in a reaction vessel at 700.0 K, its equilibrium partial pressure is 0.296 atm.Calculate Kc at 700.0 K.
A)408
B)0.123
C)0.429
D)0.141
E)2.45 *10-3
A)408
B)0.123
C)0.429
D)0.141
E)2.45 *10-3

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
29
For the equilibrium CH4 (g) +2 H2 S(g) ⇆ CS2 (g) +4 H2 (g), the concentrations at equilibrium are [CH4] = 0.3322 M, [H2S] = 0.6644 M, [CS2] =0.0678 M, and [H2] =0.2712 M at 1400.0 K.Calculate Kc.
A)0.167
B)2.50 * 10-3
C)4.00 * 102
D)6.00
E)0.694
A)0.167
B)2.50 * 10-3
C)4.00 * 102
D)6.00
E)0.694

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the following equilibrium: CH4 (g) +H2O(g) ⇄ CO(g) +3 H2 (g).When 1.00 M CH4 and 1.00 M H2O are placed in a reaction vessel at 1400.0 K, their equilibrium concentrations are 0.530 M.Calculate Kp at 1400 K.
A)4.69
B)3.55 * 10-4
C)6.19 * 104
D)539
E)0.0408
A)4.69
B)3.55 * 10-4
C)6.19 * 104
D)539
E)0.0408

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
31
The equilibrium constant for the formation of calcium carbonate from the ions in solution is 2.2 *108 according to the reaction Ca2+(aq) + CO32 - (aq) ⇄ CaCO3 (s).What is the value of the equilibrium
Constant for the reverse of this reaction?
A)the same, 2.2 *108
B)(-2.2 *108)
C)2.2 *10-8
D)4.5 * 10-9
E)4.5 *109
Constant for the reverse of this reaction?
A)the same, 2.2 *108
B)(-2.2 *108)
C)2.2 *10-8
D)4.5 * 10-9
E)4.5 *109

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
32
For the chemical equilibrium aA + bB ⇄ cC, the value of the equilibrium constant K is 10.What is the value of the equilibrium constant for the reaction 2cC ⇄ 2aA + 2bB?
A)0.1
B)0.2
C)0.01
D)20
E)10
A)0.1
B)0.2
C)0.01
D)20
E)10

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the equilibrium CL2 (g) + 2 NO(g) ⇄ 2 NOCl(g).When 2.000 M NOCl is placed in an empty tank at 500.0 K and allowed to come to equilibrium, the equilibrium concentration of Cl2 is 0.979 M.Calculate Kc.
A)45.6
B)15.2
C)0.938
D)2130
E)4.70 * 10-4
A)45.6
B)15.2
C)0.938
D)2130
E)4.70 * 10-4

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
34
Which statement below regarding the mass action expression and the equilibrium constant expression is FALSE?
A)The mass action expression is usually applied to systems that have not attained equilibrium.
B)The mass action expression and the equilibrium constant expression have the same mathematical form.
C)The mass action expression never has the same value as the equilibrium constant expression.
D)There are infinitely many values for the mass action expression at a given temperature.
E)There is only one value of the equilibrium constant expression at a given temperature.
A)The mass action expression is usually applied to systems that have not attained equilibrium.
B)The mass action expression and the equilibrium constant expression have the same mathematical form.
C)The mass action expression never has the same value as the equilibrium constant expression.
D)There are infinitely many values for the mass action expression at a given temperature.
E)There is only one value of the equilibrium constant expression at a given temperature.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
35
For the chemical equilibrium aA + bB ⇄ cC, the value of the equilibrium constant K is 10.What is the value of the equilibrium constant for the reaction 2aA+2bB ⇄ 2cC?
A)10
B)20
C)40
D)100
E)400
A)10
B)20
C)40
D)100
E)400

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
36
The law of mass action is a result of
A)the law of conservation of matter.
B)the law of conservation of energy.
C)kinetics of reversible reactions.
D)limiting reagent stoichiometry.
E)the third law of thermodynamics.
A)the law of conservation of matter.
B)the law of conservation of energy.
C)kinetics of reversible reactions.
D)limiting reagent stoichiometry.
E)the third law of thermodynamics.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
37
Two students measure the equilibrium constant for the same chemical reaction.One student finds Kc to be 130; the other calculates Kc to be 11.4.The instructor checks their results and says they are both correct.Which statement below is a plausible explanation?
A)The values vary according to the way the measurement is made.One student must have measured product concentrations whereas the second must have measured reactant concentrations.
B)The values vary according to the starting conditions of the reaction prior to equilibrium.One student must have started with all reactants whereas the second must have started with all products.
C)The values vary according to the stoichiometric coefficients that are used.The balancing coefficients that the first student used must have been twice those that the second used.
D)The values vary according to direction of the reaction.One student must have used the reverse reaction.
E)The instructor must have made a mistake, as the equilibrium constant for a reaction must always be the same.
A)The values vary according to the way the measurement is made.One student must have measured product concentrations whereas the second must have measured reactant concentrations.
B)The values vary according to the starting conditions of the reaction prior to equilibrium.One student must have started with all reactants whereas the second must have started with all products.
C)The values vary according to the stoichiometric coefficients that are used.The balancing coefficients that the first student used must have been twice those that the second used.
D)The values vary according to direction of the reaction.One student must have used the reverse reaction.
E)The instructor must have made a mistake, as the equilibrium constant for a reaction must always be the same.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
38
At room temperature an increase in the number of moles of gas as the reaction goes from reactants to products in a gas-phase equilibrium results in
A)Kp Kc.
B)Kp Kc.
C)Kp = Kc.
D)Kp + Kc =(RT) n.
E)KpKc=(RT) n.
A)Kp Kc.
B)Kp Kc.
C)Kp = Kc.
D)Kp + Kc =(RT) n.
E)KpKc=(RT) n.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
39
For the equilibrium 2 PH3 (g) ⇄ P 2 (g) +3 H2 (g), the equilibrium partial pressures are PPH= 0.022 atm, PP = 0.289 atm, and PH = 0.867 atm at 873 K.Calculate Kp. 
A)0.0585
B)17.1
C)0.0441
D)2.50* 10-3
E)389

A)0.0585
B)17.1
C)0.0441
D)2.50* 10-3
E)389

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
40
For the following hypothetical equilibrium, A +2 B ⇄ C, what is the value of the equilibrium constant if the concentrations at equilibrium are [A] = 4.5 *10-5 M, [B] = 2.2 *10-2 M, and [C] = 9.4 *10-3 M?
A)0.22
B)9.9
C)4.3 * 105
D)2.3 *108
E)9.5*103
A)0.22
B)9.9
C)4.3 * 105
D)2.3 *108
E)9.5*103

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
41
In equilibrium expressions, the concentrations of pure solids and liquids
A)have the assigned value of one.
B)have the assigned value of zero.
C)have constant values, cs and c1.
D)are determined from the density and molar mass.
E)are treated as any other solute.
A)have the assigned value of one.
B)have the assigned value of zero.
C)have constant values, cs and c1.
D)are determined from the density and molar mass.
E)are treated as any other solute.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the equilibrium CH4 (g) + H2 O(g) +206 kJ ⇄ CO(g) +3 H2 (g).Which of the following disturbances will NOT cause the system to shift to the right to reestablish equilibrium?
A)The partial pressure of CH4 increases.
B)The partial pressure of CO decreases.
C)The volume decreases.
D)The temperature increases.
E)All of these will cause the system to shift to the right.
A)The partial pressure of CH4 increases.
B)The partial pressure of CO decreases.
C)The volume decreases.
D)The temperature increases.
E)All of these will cause the system to shift to the right.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
43
In a simple equilibrium A(g) +B(g) ⇄ C(g), will there be any stress to the system if both B and C are added to the equilibrium system simultaneously and in the same amount?
A)No, these two stresses will always cancel each other out.
B)No, these two stresses will cancel each other out unless the initial concentrations of B and C are also the same.
C)Yes, the same amount of A is also required for the stresses to cancel.
D)Yes, unless the initial concentrations of B and C are also the same.
E)More than two of the above statements are correct.
A)No, these two stresses will always cancel each other out.
B)No, these two stresses will cancel each other out unless the initial concentrations of B and C are also the same.
C)Yes, the same amount of A is also required for the stresses to cancel.
D)Yes, unless the initial concentrations of B and C are also the same.
E)More than two of the above statements are correct.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the equilibrium A +B ⇄ C.What is significant about the reaction quotient when [B] = [C]?
A)only that [B] = [C]
B)[A] = [B]= [C]
C)[B] = [C] = K
D)[A]=K
E)The reaction must run in the forward direction.
A)only that [B] = [C]
B)[A] = [B]= [C]
C)[B] = [C] = K
D)[A]=K
E)The reaction must run in the forward direction.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
45
For the equilibrium N2 (g) +O2 (g) ⇄ 2 NO(g), Kp = 0.0017 at 2300 K.At a given point, the partial pressures of the gases are PN 2 =PO2 = 0.660 atm and PNO = 0.0272 atm.Which statement below is true?
A)Q K, so the reaction will continue to make more products.
B)Q K, so the reaction will consume products to make more reactants.
C)Q = K, so the system is at equilibrium.
D)The value of K will decrease until it is equal to Q.
E)The value of K will increase until it is equal to Q.
A)Q K, so the reaction will continue to make more products.
B)Q K, so the reaction will consume products to make more reactants.
C)Q = K, so the system is at equilibrium.
D)The value of K will decrease until it is equal to Q.
E)The value of K will increase until it is equal to Q.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
46
Which statement below, if any, is NOT a perturbation or stress to the equilibrium position of an endothermic chemical reaction that involves one or more gases?
A)adding reactants to a gas or solution reaction
B)removing products from a gas or solution reaction
C)decreasing the temperature
D)increasing pressure by adding an inert gas to a reaction in the gas phase
E)All of the above are perturbations to chemical equilibrium.
A)adding reactants to a gas or solution reaction
B)removing products from a gas or solution reaction
C)decreasing the temperature
D)increasing pressure by adding an inert gas to a reaction in the gas phase
E)All of the above are perturbations to chemical equilibrium.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
47
If the reaction quotient Q has a larger value than the related equilibrium constant K,
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will increase until it is equal to Q.
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will increase until it is equal to Q.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
48
For the equilibrium H2 (g) + S(s) ⇄ H2 S(g), Kc =6.1*105 at 298 K.If the concentrations of H2 and H2S are equal, which statement below is true?
A)The concentration of S equals Kc.
B)The equilibrium constant equals 1.
C)The reaction quotient equals 1.
D)The system is at equilibrium.
E)The concentrations of H2 and H2S can never be equal.
A)The concentration of S equals Kc.
B)The equilibrium constant equals 1.
C)The reaction quotient equals 1.
D)The system is at equilibrium.
E)The concentrations of H2 and H2S can never be equal.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
49
For the equilibrium 2 NO(g) +3H2O2 (g) ⇄ 2 NH3 (g) + 4O2 (g), Kp = 0.244 at 1400 K.At a given point, the partial pressures of the gases are PNO =0.0382 atm, PH2 O2 = 0.0974 atm, PNH 3 = 0.264 atm, and PO2 = 0.126 atm.Which statement below is true?
A)Q K, so the reaction will continue to make more products.
B)Q K, so the reaction will consume products to make more reactants.
C)Q= K, so the system is at equilibrium.
D)The value of K will decrease until it is equal to Q.
E)The value of K will increase until it is equal to Q.
A)Q K, so the reaction will continue to make more products.
B)Q K, so the reaction will consume products to make more reactants.
C)Q= K, so the system is at equilibrium.
D)The value of K will decrease until it is equal to Q.
E)The value of K will increase until it is equal to Q.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following occurs when products are removed from a chemical reaction in solution or the gas phase at equilibrium?
A)Q increases and the equilibrium shifts to produce more products.
B)Q increases and the equilibrium shifts to produce more reactants.
C)Q decreases and the equilibrium shifts to produce more products.
D)Q decreases and the equilibrium shifts to produce more reactants.
E)Q is unchanged by the addition of reactants.
A)Q increases and the equilibrium shifts to produce more products.
B)Q increases and the equilibrium shifts to produce more reactants.
C)Q decreases and the equilibrium shifts to produce more products.
D)Q decreases and the equilibrium shifts to produce more reactants.
E)Q is unchanged by the addition of reactants.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
51
Which statement below regarding the effect of a catalyst on chemical equilibrium is true?
A)Only the forward rate increases, so the quantity of products increases.
B)Only the forward rate increases, but the quantity of products remains the same.
C)Both the forward and reverse rates increase and the quantity of products increases.
D)Both the forward and reverse rates increase, but the quantity of products is unchanged.
E)The effect varies depending on whether the reaction is endothermic or exothermic.
A)Only the forward rate increases, so the quantity of products increases.
B)Only the forward rate increases, but the quantity of products remains the same.
C)Both the forward and reverse rates increase and the quantity of products increases.
D)Both the forward and reverse rates increase, but the quantity of products is unchanged.
E)The effect varies depending on whether the reaction is endothermic or exothermic.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
52
An equilibrium that strongly favors products has
A)a value of K 1.
B)a value of K 1.
C)a value of Q 1.
D)a value of Q 1.
E)K = Q.
A)a value of K 1.
B)a value of K 1.
C)a value of Q 1.
D)a value of Q 1.
E)K = Q.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
53
For a chemical reaction at equilibrium, which actions) below will change the value of the equilibrium constant K? I.Changing the temperature
II)Changing the total concentration of reactants and products
III)Changing the reaction coefficients
A)I only
B)II only
C)III only
D)I and II only
E)I and III only
II)Changing the total concentration of reactants and products
III)Changing the reaction coefficients
A)I only
B)II only
C)III only
D)I and II only
E)I and III only

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
54
Increasing the temperature of an exothermic reaction results in
A)more products and fewer reactants.
B)more reactants and fewer products.
C)more reactants and products.
D)fewer reactants and products.
E)no change in the quantities of reactants and products.
A)more products and fewer reactants.
B)more reactants and fewer products.
C)more reactants and products.
D)fewer reactants and products.
E)no change in the quantities of reactants and products.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following occurs when reactants are added to a chemical reaction in solution or the gas phase at equilibrium?
A)Q increases and the equilibrium shifts to produce more products.
B)Q increases and the equilibrium shifts to produce more reactants.
C)Q decreases and the equilibrium shifts to produce more products.
D)Q decreases and the equilibrium shifts to produce more reactants.
E)Q is unchanged by the addition of reactants.
A)Q increases and the equilibrium shifts to produce more products.
B)Q increases and the equilibrium shifts to produce more reactants.
C)Q decreases and the equilibrium shifts to produce more products.
D)Q decreases and the equilibrium shifts to produce more reactants.
E)Q is unchanged by the addition of reactants.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
56
For the equilibrium 2 NOBr(g) ⇄ 2 NO(g) + Br2 (g), Kc = 1.56 * 10-3 at 300 K.Suppose 0.400 M NOBr is placed in a reaction vessel at 300 K.At a given point, the concentration of Br2 is 0.0134 M.Which statement below is true?
A)Q K, so the reaction will continue to make more products.
B)Q K, so the reaction will consume products to make more reactants.
C)Q = K, so the system is at equilibrium.
D)The value of K will decrease until it is equal to Q.
E)The value of K will increase until it is equal to Q.
A)Q K, so the reaction will continue to make more products.
B)Q K, so the reaction will consume products to make more reactants.
C)Q = K, so the system is at equilibrium.
D)The value of K will decrease until it is equal to Q.
E)The value of K will increase until it is equal to Q.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
57
If the reaction quotient Q has a smaller value than the related equilibrium constant K,
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will decrease until it is equal to Q.
A)the reaction is at equilibrium.
B)the reaction will continue to make more products.
C)the reaction will consume products and make reactants.
D)the reaction will release heat to achieve equilibrium.
E)the value of K will decrease until it is equal to Q.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
58
Cylinders of NO gas may contain small quantities of oxygen as impurities, leading to the formation of NO2 in equilibrium with the NO and oxygen.Is this contamination by NO2 dependent on pressure in the tank?
A)Yes, there will be more NO2 at higher pressures.
B)Yes, there will be less NO2 at higher pressures.
C)No, the amount of NO2 has nothing to do with pressure.
D)No, the amount of NO2 depends on the partial pressure, not the total pressure.
E)There is no way to tell without additional information.
A)Yes, there will be more NO2 at higher pressures.
B)Yes, there will be less NO2 at higher pressures.
C)No, the amount of NO2 has nothing to do with pressure.
D)No, the amount of NO2 depends on the partial pressure, not the total pressure.
E)There is no way to tell without additional information.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
59
Addition of reactants to a chemical reaction in solution or gas phase at equilibrium results in
A)an increase in K and a shift in equilibrium to produce more products.
B)an increase in K and a shift in equilibrium to produce more reactants.
C)a decrease in K and a shift in equilibrium to produce more products.
D)a decrease in K and a shift in equilibrium to produce more reactants.
E)no change in K and a shift in equilibrium to produce more products.
A)an increase in K and a shift in equilibrium to produce more products.
B)an increase in K and a shift in equilibrium to produce more reactants.
C)a decrease in K and a shift in equilibrium to produce more products.
D)a decrease in K and a shift in equilibrium to produce more reactants.
E)no change in K and a shift in equilibrium to produce more products.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
60
If the temperature of an endothermic reaction at equilibrium could be increased instantaneously, what would be the instantaneous effect on Q and K before equilibrium was again achieved?
A)Q would increase and K would stay the same.
B)Q would decrease and K would stay the same.
C)Q would stay the same and K would increase.
D)Q would stay the same and K would decrease.
E)Both Q and K would stay the same.
A)Q would increase and K would stay the same.
B)Q would decrease and K would stay the same.
C)Q would stay the same and K would increase.
D)Q would stay the same and K would decrease.
E)Both Q and K would stay the same.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
61
What happens to the equilibrium between NO2g) and N2O4g) in inert argon when the volume is increased and additional argon is added to maintain a constant total pressure?
A)The ratio of NO2 to N2O4 increases solely because of the increase in volume.
B)The ratio of NO2 to N2O4 increases solely because of the addition of argon.
C)The ratio of NO2 to N2O4 decreases solely because of the increase in volume.
D)The ratio of NO2 to N2O4 decreases solely because of the addition of argon.
E)The ratio of NO2 to N2O4 remains the same, as the effects of the two processes cancel.
A)The ratio of NO2 to N2O4 increases solely because of the increase in volume.
B)The ratio of NO2 to N2O4 increases solely because of the addition of argon.
C)The ratio of NO2 to N2O4 decreases solely because of the increase in volume.
D)The ratio of NO2 to N2O4 decreases solely because of the addition of argon.
E)The ratio of NO2 to N2O4 remains the same, as the effects of the two processes cancel.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following relationships are sufficient to ensure that reactants and products will be in their standard state at equilibrium?
I. G = 0
II. G = 0
III.K = 1
A)I only
B)II only
C)III only
D)II and III only
E)Reactants and products can never be in equilibrium in their standard states.
I. G = 0
II. G = 0
III.K = 1
A)I only
B)II only
C)III only
D)II and III only
E)Reactants and products can never be in equilibrium in their standard states.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
63
For the reaction 2 H2 S(g) ⇆ 2 H2 (g) + S2 (g), Kc =1.7 * 10-7 at 800.0
C.If the initial concentration of H2S in a closed container is 0.75 M, what is the approximate equilibrium concentration of H2?
A)0.37 M
B)3.2 * 10-3 M
C)6.3 * 10-3 M
D)5.8 * 10-3 M
E)2.9 * 10-3 M
C.If the initial concentration of H2S in a closed container is 0.75 M, what is the approximate equilibrium concentration of H2?
A)0.37 M
B)3.2 * 10-3 M
C)6.3 * 10-3 M
D)5.8 * 10-3 M
E)2.9 * 10-3 M

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
64
The decomposition of NOCl to form NO and Cl2, 2 NOCl(g) ⇄ 2 NO(g) + CL2 (g), has a Kp value of 1.6 *10-5 at some temperature.If the initial partial pressures of NO and Cl2 in a closed container are 0.80 atm and 0.40 atm, respectively, what is the approximate equilibrium partial pressure of NOCl?
A)0.77 atm
B)0.79 atm
C)0.80 atm
D)0.014 atm
E)0.027 atm
A)0.77 atm
B)0.79 atm
C)0.80 atm
D)0.014 atm
E)0.027 atm

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
65
When can an x be ignored in solving an equilibrium expression derived from a RICE table?
A)whenever it simplifies the calculation
B)whenever it is very much smaller than the term it is added to or subtracted from
C)whenever the equilibrium concentration for that species is relatively very small
D)whenever it is raised to any power higher than 1
E)never
A)whenever it simplifies the calculation
B)whenever it is very much smaller than the term it is added to or subtracted from
C)whenever the equilibrium concentration for that species is relatively very small
D)whenever it is raised to any power higher than 1
E)never

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
66
The decomposition of NOCl to form NO and Cl2, 2 NOCl(g) ⇄ 2 NO(g) + CL2 (g), has a Kp value of 1.6 *10-5 at some temperature.If 0.500 atm of NOCl is placed in a closed vessel and allowed to come to equilibrium, what is its approximate equilibrium partial pressure?
A)0.013 atm
B)0.025 atm
C)0.48 atm
D)0.49 atm
E)0.50 atm
A)0.013 atm
B)0.025 atm
C)0.48 atm
D)0.49 atm
E)0.50 atm

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
67
The reaction of hydrogen gas with chlorine gas, H2 g) + CL2 g) 2 HClg), has a Kp value of 0.500.If 0.250 atm of both H2 and Cl2 are placed in a closed vessel and allowed to come to equilibrium, what is the equilibrium partial pressure of HClg)?
A)0.250 atm
B)0.104 atm
C)0.0653 atm
D)0.177 atm
E)0.131 atm
A)0.250 atm
B)0.104 atm
C)0.0653 atm
D)0.177 atm
E)0.131 atm

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following must be true for a reaction to proceed to form products?
A)Q K, G 0, and G 0
B)Q K and G 0; G can be anything
C)Q K and G 0; G can be anything
D)Q K, G 0, and G 0
E)Q K and G 0; G can be anything
A)Q K, G 0, and G 0
B)Q K and G 0; G can be anything
C)Q K and G 0; G can be anything
D)Q K, G 0, and G 0
E)Q K and G 0; G can be anything

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
69
The fluoride ion, F-, reacts with water as a weak base: F-(aq) + H2 O(l) ⇄ HF(aq) + OH-(aq), with K = 1.5 * 10-11 at 25
C.If sodium fluoride were dissolved in water to make a 0.150 M solution, what would be the resulting concentration of OH-?
A)0.15 M
B)1.5 * 10-6 M
C)1.1 * 10-12 M
D)5.8* 10-7 M
E)2.3 * 10-12 M
C.If sodium fluoride were dissolved in water to make a 0.150 M solution, what would be the resulting concentration of OH-?
A)0.15 M
B)1.5 * 10-6 M
C)1.1 * 10-12 M
D)5.8* 10-7 M
E)2.3 * 10-12 M

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
70
If K for a reaction is large,
A)( G is definitely negative.)
B)( G is definitely positive.)
C)( G is equal to zero.)
D)( G is definitely negative.)
E)( G is definitely positive.)
A)( G is definitely negative.)
B)( G is definitely positive.)
C)( G is equal to zero.)
D)( G is definitely negative.)
E)( G is definitely positive.)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the choices below contains quantities that may have only positive values?
A)K and Q
B)( G, H, and S)
C)ln K and ln Q
D)K and G
E)none of the above
A)K and Q
B)( G, H, and S)
C)ln K and ln Q
D)K and G
E)none of the above

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
72
The reaction of bromine gas with chlorine gas, Br2 (g) + CL2 (g) ⇄ 2 BrCl(g), has a Kp value of 7.20.If the initial partial pressures of Br2, Cl2, and BrCl in a closed container are all 0.500 atm, what is the equilibrium partial pressure of BrCl(g)?
A)0.500 atm
B)0.680 atm
C)0.029 atm
D)0.859 atm
E)0.987 atm
A)0.500 atm
B)0.680 atm
C)0.029 atm
D)0.859 atm
E)0.987 atm

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
73
For a particular hypothetical reaction, 2A(aq) +B(aq) C(aq), the value of G is 2.50 * 102 kJ/mol.What is the value of G for this reaction at 298 K when [A] = 0.600 M, [B]= 0.100 M, and [C]= 4.00 *10-3 M ?
A)2.48 * 102 kJ/mol
B)2.45 * 102 kJ/mol
C)2.78* 103 kJ/mol
D)2.50 *102 kJ/mol
E)2.55* 102 kJ/mol
A)2.48 * 102 kJ/mol
B)2.45 * 102 kJ/mol
C)2.78* 103 kJ/mol
D)2.50 *102 kJ/mol
E)2.55* 102 kJ/mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
74
A reaction X + 2Y ⇄ 3 Z is started with 1.0 M Z and no X or Y.To calculate the equilibrium concentrations of all species using a RICE table, which of the following would you enter in the Z column for the C row?
A)1.0 M
B)1.0 M - x
C)1.0 M - 3x
D)1.0 M + 3x
E)(-3x)
A)1.0 M
B)1.0 M - x
C)1.0 M - 3x
D)1.0 M + 3x
E)(-3x)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
75
A sketch of the free energy for a hypothetical chemical equilibrium is shown here.What part of the plot on the axis representing the relative quantities of reactants and products corresponds to a value of G that is greater than zero? 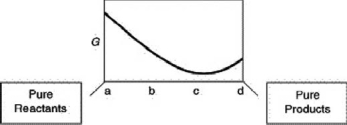
A)a to c
B)c only
C)c to d
D)b to d
E)none of these

A)a to c
B)c only
C)c to d
D)b to d
E)none of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
76
For the gas-phase oxidation of sulfur dioxide, 2 SO2 (g) +O2 (g) ⇄ 2 SO3 (g), Kp = 4.17 * 102 at 200.0
C.If a closed vessel is filled with SO3 at an initial pressure of 0.033 atm at 200
C, what is the equilibrium partial pressure of SO3?
A)more than 0.033 atm
B)less than 0.033 atm
C)0.033 atm exactly
D)equal to 0.033/4.17 * 102
E)More information is required.
C.If a closed vessel is filled with SO3 at an initial pressure of 0.033 atm at 200
C, what is the equilibrium partial pressure of SO3?
A)more than 0.033 atm
B)less than 0.033 atm
C)0.033 atm exactly
D)equal to 0.033/4.17 * 102
E)More information is required.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
77
A perturbation or stress to a chemical reaction at equilibrium
A)increases the free energy of the system.
B)decreases the free energy of the system.
C)neither increases nor decreases the free energy of the system.
D)may increase or decrease the free energy of the system depending on the nature of the stress.
E)causes the free energy of the system to become zero.
A)increases the free energy of the system.
B)decreases the free energy of the system.
C)neither increases nor decreases the free energy of the system.
D)may increase or decrease the free energy of the system depending on the nature of the stress.
E)causes the free energy of the system to become zero.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
78
For the reaction 2 H2 S(g) ⇆ 2 H2 (g) + S2 (g), Kp =1.5 * 10-5 at 800.0
C.If the initial partial pressures of H2 and S2 in a closed container are 4.00 atm and 2.00 atm, respectively, what is the approximate equilibrium partial pressure of H2S?
A)0.0783 atm
B)0.0493 atm
C)0.0247 atm
D)3.95 atm
E)3.92 atm
C.If the initial partial pressures of H2 and S2 in a closed container are 4.00 atm and 2.00 atm, respectively, what is the approximate equilibrium partial pressure of H2S?
A)0.0783 atm
B)0.0493 atm
C)0.0247 atm
D)3.95 atm
E)3.92 atm

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
79
In the equation relating equilibrium to thermodynamics, a - =RTb,
A)a = G and b = ln K.
B)a = G and b = K.
C)a = G= and b =ln K.
D)a = G= and b = K.
E)a = ln G= and b = K.
A)a = G and b = ln K.
B)a = G and b = K.
C)a = G= and b =ln K.
D)a = G= and b = K.
E)a = ln G= and b = K.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
80
What is the value of the equilibrium constant at 462 K for a chemical equilibrium that has a H value of=46.0 kJ/mol and a S value of =92.0 J/mol · K?
A)1.0 * 1010
B)1.6 * 10-5
C)0.40
D)2.5
E)6.3 * 104
A)1.0 * 1010
B)1.6 * 10-5
C)0.40
D)2.5
E)6.3 * 104

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck


