Deck 15: Acid-Base Equilibria Proton Transfer in Biological Systems
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/123
Play
Full screen (f)
Deck 15: Acid-Base Equilibria Proton Transfer in Biological Systems
1
Which of the following is a strong acid?
A)nitrous acid, HNO2
B)sulfurous acid, H2SO3
C)carbonic acid, H2CO3
D)hydrofluoric acid, HF
E)perchloric acid, HClO4
A)nitrous acid, HNO2
B)sulfurous acid, H2SO3
C)carbonic acid, H2CO3
D)hydrofluoric acid, HF
E)perchloric acid, HClO4
perchloric acid, HClO4
2
Which of the following is NOT a strong base?
A)lithium hydroxide, LiOH
B)sodium hydroxide, NaOH
C)potassium hydroxide, KOH
D)strontium hydroxide, SrOH)2
E)ammonium hydroxide, NH4OH
A)lithium hydroxide, LiOH
B)sodium hydroxide, NaOH
C)potassium hydroxide, KOH
D)strontium hydroxide, SrOH)2
E)ammonium hydroxide, NH4OH
ammonium hydroxide, NH4OH
3
Three acids found in foods are lactic acid (in milk products), oxalic acid (in rhubarb), and malic acid (in apples).The pKa values are LA = 3.88, OA =1.23, and MA = 3.40.Which list has these acids in order of decreasing acid strength?
A)LA OA MA
B)LA MA OA
C)OA MA LA
D)OA LA MA
E)MA LA OA
A)LA OA MA
B)LA MA OA
C)OA MA LA
D)OA LA MA
E)MA LA OA
OA MA LA
4
Which of the following is a strong base?
A)CH3NH2
B)BaOH)2
C)H2O2
D)CrOH)3
E)CH3COOH
A)CH3NH2
B)BaOH)2
C)H2O2
D)CrOH)3
E)CH3COOH

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
5
In the Brønsted-Lowry definition of acids and bases, an acid
A)is a proton donor.
B)is a proton acceptor.
C)forms stable hydrogen bonds.
D)breaks stable hydrogen bonds.
E)corrodes metals.
A)is a proton donor.
B)is a proton acceptor.
C)forms stable hydrogen bonds.
D)breaks stable hydrogen bonds.
E)corrodes metals.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
6
In the Brønsted-Lowry definition of acids and bases, a base
A)is a proton donor.
B)is a proton acceptor.
C)forms stable hydrogen bonds.
D)breaks stable hydrogen bonds.
E)corrodes metals.
A)is a proton donor.
B)is a proton acceptor.
C)forms stable hydrogen bonds.
D)breaks stable hydrogen bonds.
E)corrodes metals.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
7
Three acids found in foods are lactic acid (in milk products), oxalic acid (in rhubarb), and malic acid (in apples).The pKa values are LA = 3.88, OA = 1.23, and MA = 3.40.Which list has the conjugate bases of these acids in order of decreasing strength?
A)lactate oxalate malate
B)oxalate malate lactate
C)lactate malate oxalate
D)oxalate lactate malate
E)malate lactate oxalate
A)lactate oxalate malate
B)oxalate malate lactate
C)lactate malate oxalate
D)oxalate lactate malate
E)malate lactate oxalate

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements regarding acid and base ionization in water is NOT correct?
A)A hydrogen atom directly bonded to an oxygen atom in a molecule is always significantly more acidic than water.
B)In strong acids, the acidic protons are generally bonded to highly electronegative elements such as O, Cl, Br, or I.
C)When a hydrogen atom is released as an H+ ion from an electronegative atom in an acid, the bonding pair of electrons remains on the electronegative atom.
D)Ionization of a strong acid in water is assisted by the strong dipole-dipole interactions between its ionizable H atoms and the O atoms in water molecules.
E)Ionization of water to form hydroxide in the presence of a base is often assisted by strong dipole-dipole interactions between H atoms in water and a lone pair of electrons in the base.
A)A hydrogen atom directly bonded to an oxygen atom in a molecule is always significantly more acidic than water.
B)In strong acids, the acidic protons are generally bonded to highly electronegative elements such as O, Cl, Br, or I.
C)When a hydrogen atom is released as an H+ ion from an electronegative atom in an acid, the bonding pair of electrons remains on the electronegative atom.
D)Ionization of a strong acid in water is assisted by the strong dipole-dipole interactions between its ionizable H atoms and the O atoms in water molecules.
E)Ionization of water to form hydroxide in the presence of a base is often assisted by strong dipole-dipole interactions between H atoms in water and a lone pair of electrons in the base.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is a strong acid?
A)HNO3
B)H2S
C)HNO2
D)HCO3-
E)HOCl
A)HNO3
B)H2S
C)HNO2
D)HCO3-
E)HOCl

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
10
Given the following Ka values, which of the following acids has the strongest conjugate base in water? benzoic, C6H5COOH Ka 6.5 105 chlorous, HClO2 Ka 1.1 102
Iodic, HIO3 Ka 1.7 101 acrylic, CH2 CHCOOH Ka 5.5 105 cyanic, HCNO Ka 3.5 104
A)benzoic
B)chlorous
C)iodic
D)acrylic
E)cyanic
Iodic, HIO3 Ka 1.7 101 acrylic, CH2 CHCOOH Ka 5.5 105 cyanic, HCNO Ka 3.5 104
A)benzoic
B)chlorous
C)iodic
D)acrylic
E)cyanic

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
11
Which of these is a strong acid that ionizes to make a weak acid?
A)H2SO3
B)H2SO4
C)H3PO4
D)HNO3
E)HCl
A)H2SO3
B)H2SO4
C)H3PO4
D)HNO3
E)HCl

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a strong acid?
A)HClO
B)HClO2
C)HClO3
D)HClO4
E)None of these is a strong acid.
A)HClO
B)HClO2
C)HClO3
D)HClO4
E)None of these is a strong acid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
13
Given the following Ka values, which of the following acids is the strongest in water? benzoic, C6H5COOH Ka 6.5 105 chlorous, HClO2 Ka 1.1 102
Iodic, HIO3 Ka 1.7 101 acrylic, CH2 CHCOOH Ka 5.5 105 cyanic, HCNO Ka 3.5 104
A)benzoic
B)chlorous
C)iodic
D)acrylic
E)cyanic
Iodic, HIO3 Ka 1.7 101 acrylic, CH2 CHCOOH Ka 5.5 105 cyanic, HCNO Ka 3.5 104
A)benzoic
B)chlorous
C)iodic
D)acrylic
E)cyanic

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
14
Use the following acid ionization constants to identify the correct decreasing order of base strengths.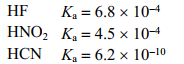
A)CN- NO2- F-
B)NO2- F- CN-
C)F- CN- NO2-
D)F- NO2- CN-
E)NO2- CN- F-
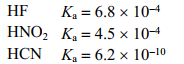
A)CN- NO2- F-
B)NO2- F- CN-
C)F- CN- NO2-
D)F- NO2- CN-
E)NO2- CN- F-

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
15
The degree of ionization of a strong acid is
A)100%.
B)between 1% and 10%.
C)between 10% and 100%.
D)dependent on the concentration of the acid.
E)dependent the value of Ka.
A)100%.
B)between 1% and 10%.
C)between 10% and 100%.
D)dependent on the concentration of the acid.
E)dependent the value of Ka.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is NOT a strong acid?
A)nitric acid, HNO3
B)sulfuric acid, H2SO4
C)carbonic acid, H2CO3
D)hydrochloric acid, HCl
E)perchloric acid, HClO4
A)nitric acid, HNO3
B)sulfuric acid, H2SO4
C)carbonic acid, H2CO3
D)hydrochloric acid, HCl
E)perchloric acid, HClO4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
17
The degree of ionization of a weak acid
I.varies with the concentration of the acid.
II.depends on the value of Ka.
III.is 100%.
IV.is greater than 50% but less than 100%.
A)I only
B)II only
C)III only
D)both I and II
E)IV only
I.varies with the concentration of the acid.
II.depends on the value of Ka.
III.is 100%.
IV.is greater than 50% but less than 100%.
A)I only
B)II only
C)III only
D)both I and II
E)IV only

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement about nitrous acid and nitric acid is correct?
A)They are both weak acids.
B)They are both strong acids.
C)They both have one ionizable proton.
D)Nitrous acid has the formula HNO3.
E)Nitric acid has the formula HNO2.
A)They are both weak acids.
B)They are both strong acids.
C)They both have one ionizable proton.
D)Nitrous acid has the formula HNO3.
E)Nitric acid has the formula HNO2.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is a strong acid?
A)H2SO3
B)H3PO4
C)HBrO
D)HF
E)HNO3
A)H2SO3
B)H3PO4
C)HBrO
D)HF
E)HNO3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is NOT correct?
A)A strong acid solution has a higher concentration than a weak acid solution.
B)A strong acid is ionized to a greater extent than a weak acid.
C)Hydrochloric acid is an example of a strong acid.
D)Acetic acid is an example of a weak acid.
E)The pH of a 0.1 M solution of acetic acid is higher than the pH of a 0.1 M solution of hydrochloric acid.
A)A strong acid solution has a higher concentration than a weak acid solution.
B)A strong acid is ionized to a greater extent than a weak acid.
C)Hydrochloric acid is an example of a strong acid.
D)Acetic acid is an example of a weak acid.
E)The pH of a 0.1 M solution of acetic acid is higher than the pH of a 0.1 M solution of hydrochloric acid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
21
Ammonia (NH3) acts as a weak base in aqueous solution.What is the acid that reacts with this base when ammonia is dissolved in water?
A)None; there are no acids in pure water.
B)H2O
C)NH4+
D)OH-
E)oxygen that always is dissolved in water
A)None; there are no acids in pure water.
B)H2O
C)NH4+
D)OH-
E)oxygen that always is dissolved in water

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements regarding the leveling effect is NOT correct?
A)All strong acids completely ionize in water and appear to have the same strength.
B)All strong bases completely hydrolyze in water and appear to have the same strength.
C)The strongest base that can exist in water is OH-.
D)The strongest acid that can exist in water is H3O+.
E)All strong acids and bases are equally strong, even in solvents other than water.
A)All strong acids completely ionize in water and appear to have the same strength.
B)All strong bases completely hydrolyze in water and appear to have the same strength.
C)The strongest base that can exist in water is OH-.
D)The strongest acid that can exist in water is H3O+.
E)All strong acids and bases are equally strong, even in solvents other than water.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
23
Suppose an aqueous solution initially contains one mole of hydrochloric acid and one mole of ammonia.Which of the following statements best describes the solution once it comes to equilibrium?
A)The solution is neutral because the ammonia neutralizes the hydrochloric acid.
B)The solution is highly acidic due to the ionization of HCl: HCl(aq) H+(aq) + Cl-(aq).
C)The solution is slightly acidic due to the equilibrium NH4+(aq) ⇄ NH3 (aq) H+(aq).
D)The solution is slightly basic due to the equilibrium NH3 (aq)+ H2O(l) ⇄ NH4+ (aq) +OH-(aq).
E)The solution is slightly basic due to the equilibrium Cl-(aq) + H2O(l) ⇄ HCl(aq) +OH-(aq).
A)The solution is neutral because the ammonia neutralizes the hydrochloric acid.
B)The solution is highly acidic due to the ionization of HCl: HCl(aq) H+(aq) + Cl-(aq).
C)The solution is slightly acidic due to the equilibrium NH4+(aq) ⇄ NH3 (aq) H+(aq).
D)The solution is slightly basic due to the equilibrium NH3 (aq)+ H2O(l) ⇄ NH4+ (aq) +OH-(aq).
E)The solution is slightly basic due to the equilibrium Cl-(aq) + H2O(l) ⇄ HCl(aq) +OH-(aq).

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a conjugate acid-base pair?
A)NaF and F-
B)HNO3 and HNO2
C)HI and I-
D)NH4+ and NH2-
E)H2O and H2O2
A)NaF and F-
B)HNO3 and HNO2
C)HI and I-
D)NH4+ and NH2-
E)H2O and H2O2

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
25
In the following reaction in aqueous solution, the acid reactant is _______ and its conjugate base product is _______. CH3 COOH(aq) +NH3 (aq) ⇄ CH3COO-(aq) +NH4 + (aq)
A)CH3COOH; CH3COO-
B)CH3COOH; NH4+
C)NH3; CH3COO-
D)NH3; NH4+
E)CH3COOH; H3O+
A)CH3COOH; CH3COO-
B)CH3COOH; NH4+
C)NH3; CH3COO-
D)NH3; NH4+
E)CH3COOH; H3O+

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
26
When pure water autoionizes, which species are produced?
A)O2-, OH-, H3O+, and H2O+
B)OH- and H3O+
C)O2- and H4O2+
D)H+ and OH-
E)2 H+ and O2-
A)O2-, OH-, H3O+, and H2O+
B)OH- and H3O+
C)O2- and H4O2+
D)H+ and OH-
E)2 H+ and O2-

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is correct?
A)K2SO3 is a stronger base than KHSO3.
B)K2CO3 is a weaker base than KHCO3.
C)NaHSO3 is a stronger acid than NaHSO4.
D)Na2HPO4 is a weaker base than NaH2PO4.
E)All of these statements are correct.
A)K2SO3 is a stronger base than KHSO3.
B)K2CO3 is a weaker base than KHCO3.
C)NaHSO3 is a stronger acid than NaHSO4.
D)Na2HPO4 is a weaker base than NaH2PO4.
E)All of these statements are correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is NOT a conjugate acid-base pair?
A)NH3 and NH4+
B)H3O+ and OH-
C)H2PO4- and HPO42-
D)HS- and H2S
E)NH3 and NH2-
A)NH3 and NH4+
B)H3O+ and OH-
C)H2PO4- and HPO42-
D)HS- and H2S
E)NH3 and NH2-

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
29
Which ending to the statement is NOT correct? The weak acid HY will be stronger than the weak acid HZ if
A)more Lewis resonance structures can be written for the Y ion than for the Z ion.
B)Y is more electronegative than Z.
C)the Y ion has more oxygen atoms than the Z ion.
D)the H-Y bond is weaker than the H-Z bond.
E)the H-Y bond is less polar than the H-Z bond.
A)more Lewis resonance structures can be written for the Y ion than for the Z ion.
B)Y is more electronegative than Z.
C)the Y ion has more oxygen atoms than the Z ion.
D)the H-Y bond is weaker than the H-Z bond.
E)the H-Y bond is less polar than the H-Z bond.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following cannot be a Brønsted-Lowry base?
A)OH-
B)H2O
C)NH3
D)NH4+
E)SH-
A)OH-
B)H2O
C)NH3
D)NH4+
E)SH-

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
31
In each of the following, the stronger acid is identified and an explanation is given.All except one are correct statements.Which one is NOT correct?
A)HCl HF, because the HF bond energy is larger than the HCl bond energy.
B)HCO3- H2CO3, because the negative charge stabilizes the loss of a proton.
C)CCl3COOH CH3COOH, because electronegative substituents stabilize the conjugate base.
D)HBrO3 HBrO2, because of the additional electronegative oxygen atom.
E)ClOH BrOH, because Cl is more electronegative than Br.
A)HCl HF, because the HF bond energy is larger than the HCl bond energy.
B)HCO3- H2CO3, because the negative charge stabilizes the loss of a proton.
C)CCl3COOH CH3COOH, because electronegative substituents stabilize the conjugate base.
D)HBrO3 HBrO2, because of the additional electronegative oxygen atom.
E)ClOH BrOH, because Cl is more electronegative than Br.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is NOT correct?
A)HClO4 is a stronger acid than HClO3.
B)HClO is a stronger acid than HCl.
C)HClO2 is a weaker acid than HClO3.
D)ClO-is a stronger base than ClO2-.
E)ClO2- is a stronger base than Cl-.
A)HClO4 is a stronger acid than HClO3.
B)HClO is a stronger acid than HCl.
C)HClO2 is a weaker acid than HClO3.
D)ClO-is a stronger base than ClO2-.
E)ClO2- is a stronger base than Cl-.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements regarding acidity, basicity, pH, and pOH is NOT correct?
A)[H+]= [OH-] in a neutral solution, and the pH = 7 at 25°C.
B)[H+] [OH-] in an acidic solution, and the pH 7 at 25°C.
C)[H+] [OH-] in a basic solution, and the pH 7 at 25°C.
D)If [H+] increases in a solution, [OH-] must decrease.
E)If the pH of a solution increases, its [H+] increases.
A)[H+]= [OH-] in a neutral solution, and the pH = 7 at 25°C.
B)[H+] [OH-] in an acidic solution, and the pH 7 at 25°C.
C)[H+] [OH-] in a basic solution, and the pH 7 at 25°C.
D)If [H+] increases in a solution, [OH-] must decrease.
E)If the pH of a solution increases, its [H+] increases.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
34
Which expression defines the autoionization constant for water, Kw?
A)[H3O+][OH-]
B)[H2O][H3O+]
C)[OH-][H2O]
D)[H4O2-][O2-]
E)[H2O][H2O]
A)[H3O+][OH-]
B)[H2O][H3O+]
C)[OH-][H2O]
D)[H4O2-][O2-]
E)[H2O][H2O]

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following lists the conjugate acid of HAsO42- first and its conjugate base second?
A)H3O-; AsO43-
B)AsO43-; H2AsO4 -
C)H3AsO4; AsO43-
D)AsO43-; H3AsO4
E)H2AsO4-; AsO43-
A)H3O-; AsO43-
B)AsO43-; H2AsO4 -
C)H3AsO4; AsO43-
D)AsO43-; H3AsO4
E)H2AsO4-; AsO43-

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is a conjugate acid-base pair?
A)H3PO4 and HPO42-
B)H2 and H-
C)CO32-and H2CO3
D)OH- and H3O-
E)H2PO4- and PO4-
A)H3PO4 and HPO42-
B)H2 and H-
C)CO32-and H2CO3
D)OH- and H3O-
E)H2PO4- and PO4-

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
37
In the following reaction in aqueous solution, the acid reactant is _______ and the base reactant is _______.
OH-(aq) +C6H5 NH3 +(aq) ⇄ C6 H5 NH2 (aq) F+H2O(l)
A)H2O; OH-
B)C6H5NH3F1+; C6H5NH2
C)H2O; C6H5NH2
D)C6H5NH3+; OH-
E)H2O; C6H5NH3+
OH-(aq) +C6H5 NH3 +(aq) ⇄ C6 H5 NH2 (aq) F+H2O(l)
A)H2O; OH-
B)C6H5NH3F1+; C6H5NH2
C)H2O; C6H5NH2
D)C6H5NH3+; OH-
E)H2O; C6H5NH3+

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is NOT related to the water autoionization constant, Kw?
A)[H3O+] [OH-]
B)1.0 *10-14 at 25°C
C)2H2O ⇄ H O++OH3-
D)pH= 7 at 25°C
E)All are related to Kw.
A)[H3O+] [OH-]
B)1.0 *10-14 at 25°C
C)2H2O ⇄ H O++OH3-
D)pH= 7 at 25°C
E)All are related to Kw.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
39
Which statement is NOT correct? Pure water at 25°C has
A)Kw = 1.0 *10-14.
B)pOH = 7.
C)[H3O+] =[OH-].
D)pH = 7.
E)All are correct.
A)Kw = 1.0 *10-14.
B)pOH = 7.
C)[H3O+] =[OH-].
D)pH = 7.
E)All are correct.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following statements is NOT correct?
A)HIO is a stronger acid than HClO.
B)HClO is a stronger acid than HBrO.
C)HClO3 is a stronger acid than HClO2.
D)HPO42- is a weaker acid than H2PO4 -.
E)H2SO4 is a stronger acid than H2SO3.
A)HIO is a stronger acid than HClO.
B)HClO is a stronger acid than HBrO.
C)HClO3 is a stronger acid than HClO2.
D)HPO42- is a weaker acid than H2PO4 -.
E)H2SO4 is a stronger acid than H2SO3.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
41
Sometimes liquid ammonia, NH3, is used as a solvent rather than water.Which expression defines the ammonia autoionization counterpart of Kw?
A)[H3O+][OH-]
B)[NH3][NH4+]
C)[NH2-][NH4+]
D)[H3O+][NH2-0]
E)[NH4+][OH-]
A)[H3O+][OH-]
B)[NH3][NH4+]
C)[NH2-][NH4+]
D)[H3O+][NH2-0]
E)[NH4+][OH-]

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
42
When [H+] =1.0* 10-7 M in water at 25°C, then
A)pH = 1.
B)pH =10-7.
C)[OH-] = 1.0 * 10-7 M.
D)[OH-] = 1.0 *107 M.
E)[OH-] = 0 M.
A)pH = 1.
B)pH =10-7.
C)[OH-] = 1.0 * 10-7 M.
D)[OH-] = 1.0 *107 M.
E)[OH-] = 0 M.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
43
When [H+] = 4.0 *10-9 M in water at 25°C, then
A)pH = 9.40.
B)pH = 7.00.
C)pH= -8.40.
D)pH = 8.40.
E)pH = -9.40.
A)pH = 9.40.
B)pH = 7.00.
C)pH= -8.40.
D)pH = 8.40.
E)pH = -9.40.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
44
The pH of a popular soft drink is 3.40; what is its hydronium ion concentration?
A)5.0* 10-4 M
B)4.0 *10-4 M
C)2.5* 103 M
D)1.0 F* 10-7 M
E)5.0 * 10-5 M
A)5.0* 10-4 M
B)4.0 *10-4 M
C)2.5* 103 M
D)1.0 F* 10-7 M
E)5.0 * 10-5 M

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
45
In evaluating the pH of an aqueous weak acid solution, usually can be ignored.
A)the concentration of the weak acid
B)the concentration of hydronium ions produced by the autoionization of water
C)the reaction of the weak acid with water
D)the concentration of ionized hydronium ion
E)the concentration of the conjugate base
A)the concentration of the weak acid
B)the concentration of hydronium ions produced by the autoionization of water
C)the reaction of the weak acid with water
D)the concentration of ionized hydronium ion
E)the concentration of the conjugate base

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
46
Boric acid is frequently used as an eyewash to treat eye infections.The pH of a 0.050 M boric acid solution is 5.28.What is the value of Ka?
A)5.3 * 10-6
B)5.5 *10-10
C)5.4* 10-8
D)5.8 * 10-4
E)5.3* 10-12
A)5.3 * 10-6
B)5.5 *10-10
C)5.4* 10-8
D)5.8 * 10-4
E)5.3* 10-12

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
47
A solution with a pOH of 6.92 has an [OH-] of
A)1.2 *10-7 M.
B)9.2 * 10-6 M.
C)6.8 * 10-6 M.
D)7.1 M.
E)6.9 M.
A)1.2 *10-7 M.
B)9.2 * 10-6 M.
C)6.8 * 10-6 M.
D)7.1 M.
E)6.9 M.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
48
The base ionization constant Kb describes which of the following reactions for a weak base, B, in aqueous solution?
A)B(aq) + H-(aq) ⇄ BH+(aq)
B)B(aq) +H3O+(aq) ⇄ BH+(aq) + H2O(l)
C)B(aq) + H2O(l) ⇄ BH+(aq) + OH--(aq)
D)B(aq) + OH-(aq) ⇄ BH+(aq) + O2 -(aq)
E)BH+ (aq)+OH-(aq)⇄ B(aq) + H2O(l)
A)B(aq) + H-(aq) ⇄ BH+(aq)
B)B(aq) +H3O+(aq) ⇄ BH+(aq) + H2O(l)
C)B(aq) + H2O(l) ⇄ BH+(aq) + OH--(aq)
D)B(aq) + OH-(aq) ⇄ BH+(aq) + O2 -(aq)
E)BH+ (aq)+OH-(aq)⇄ B(aq) + H2O(l)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
49
Pure water at any temperature has
A)a pH less than 7.
B)a pOH more than 7.
C)[H3O+] =[OH-].
D)pH= 7.
E)no hydronium ions in it.
A)a pH less than 7.
B)a pOH more than 7.
C)[H3O+] =[OH-].
D)pH= 7.
E)no hydronium ions in it.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
50
Carbon dioxide in the atmosphere in rainwater often lowers the pH to 5.0-5.6.The rainwater is
A)neutral.
B)acidic.
C)basic.
D)gray.
E)poisonous.
A)neutral.
B)acidic.
C)basic.
D)gray.
E)poisonous.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
51
A cup of coffee has a hydroxide ion concentration of 1 * 10-10 M.What is the pH of this coffee?
A)1.0*10-4
B)4.0
C)10.0
D)7.0
E)(-10.0)
A)1.0*10-4
B)4.0
C)10.0
D)7.0
E)(-10.0)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
52
Suppose 0.035 moles of HCl is dissolved in enough water to produce 750 mL of solution.What is the pH?
A)pH =4.33
B)pH = 7.00
C)pH = 1.58
D)pH = -4.33
E)pH =1.33
A)pH =4.33
B)pH = 7.00
C)pH = 1.58
D)pH = -4.33
E)pH =1.33

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
53
A 0.270 M monoprotic weak acid solution has a pH of 2.50.What is the pKa of this acid?
A)4.43
B)1.93
C)5.57
D)9.57
E)8.07
A)4.43
B)1.93
C)5.57
D)9.57
E)8.07

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
54
Suppose 0.50 L of a HNO3 solution has a pH of 3.30.How many moles of HNO3 must have been initially dissolved in the solution?
A)2.5 *10-4 moles
B)5.0 *10-4 moles
C)1.7 * 10-3 moles
D)1.0 * 10-3 moles
E)1.8 * 10-2 moles
A)2.5 *10-4 moles
B)5.0 *10-4 moles
C)1.7 * 10-3 moles
D)1.0 * 10-3 moles
E)1.8 * 10-2 moles

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
55
A solution with a pOH of 11.70 has an [H+] of
A)2.0 *10-12 M.
B)3.2 *10-4 M.
C)0.36 M.
D)5.0 * 10-3 M.
E)2.30 M.
A)2.0 *10-12 M.
B)3.2 *10-4 M.
C)0.36 M.
D)5.0 * 10-3 M.
E)2.30 M.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
56
A solution with a pH of 9.50 has a pOH of
A)9.50.
B)0.50.
C)4.50.
D)23.5.
E)19.0.
A)9.50.
B)0.50.
C)4.50.
D)23.5.
E)19.0.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
57
The pH of a 0.250 M hypoiodous (HOI) solution is 5.620.What is the value of Ka?
A)5.76* 10-12
B)2.40 * 10-6
C)2.30 *10-11
D)4.17* 10-9
E)6.95 *10-17
A)5.76* 10-12
B)2.40 * 10-6
C)2.30 *10-11
D)4.17* 10-9
E)6.95 *10-17

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
58
The acid ionization equilibrium constant, Ka, describes which of the following reactions where HA is a generic weak acid)?
A)HA(aq) +OH sup>- (aq) → H2O(l) + A-(aq)
B)HA(aq) + H2O(l) → H3O+(aq) + A-(aq)
C)HA(aq) + H3 O+(aq) → H2 A+(aq) + H2 O(l)
D)HA(aq) + H2 A+(aq) → H2 A+(aq) +HA(aq)
E)H3O+(aq) +A-(aq) → HA(aq) + H2O(l)
A)HA(aq) +OH sup>- (aq) → H2O(l) + A-(aq)
B)HA(aq) + H2O(l) → H3O+(aq) + A-(aq)
C)HA(aq) + H3 O+(aq) → H2 A+(aq) + H2 O(l)
D)HA(aq) + H2 A+(aq) → H2 A+(aq) +HA(aq)
E)H3O+(aq) +A-(aq) → HA(aq) + H2O(l)

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following shows a relationship expressed in the correct number of significant figures?
A)[H+] = 1.270*10-2 M; pH =1.896
B)pH = 2.273; [H+] = 5.333 *10-3 M
C)[H+] = 1.80 *10-2 M; pH = 1.74
D)pH =8.9; [H+] = 1.3*10-9 M
E)pH =4.0; [H+] = 1 *10-4 M
A)[H+] = 1.270*10-2 M; pH =1.896
B)pH = 2.273; [H+] = 5.333 *10-3 M
C)[H+] = 1.80 *10-2 M; pH = 1.74
D)pH =8.9; [H+] = 1.3*10-9 M
E)pH =4.0; [H+] = 1 *10-4 M

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
60
In an experiment, 0.438 L of 0.152 M NaOH is diluted with water to a final volume of 3.00 L.What is the pH of the dilute solution?
A)1.654
B)13.182
C)1.295
D)12.705
E)12.346
A)1.654
B)13.182
C)1.295
D)12.705
E)12.346

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
61
What is the hydronium ion concentration of a 0.20 M diethylamine solution? (Kb value for diethylamine = 8.6*10-4)
A)1.2 *10-11 M
B)6.6 * 10-9 M
C)1.5 *10-6 M
D)7.6* 10-13 M
E)1.3 *10-2 M
A)1.2 *10-11 M
B)6.6 * 10-9 M
C)1.5 *10-6 M
D)7.6* 10-13 M
E)1.3 *10-2 M

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
62
What is the pH of a 0.0250 M BaOH)2 solution?
A)1.602
B)1.301
C)11.004
D)12.398
E)12.700
A)1.602
B)1.301
C)11.004
D)12.398
E)12.700

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
63
What is the hydronium ion concentration of a 0.010 M acetic acid solution? (Ka for acetic acid = 1.76 *10-5)
A)1.8 * 10-3
B)1.8 * 10-5
C)1.0 * 10-2
D)1.8 * 10-7
E)4.2 *0-4
A)1.8 * 10-3
B)1.8 * 10-5
C)1.0 * 10-2
D)1.8 * 10-7
E)4.2 *0-4

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
64
Piperidine, C5H11N, is a weak base in water.If a 0.120 M piperidine solution has a pH of 12.077, what is the Kb of piperidine?
A)4.78 *10-2
B)2.98* 10-3
C)1.19 * 10-3
D)1.31 * 10-3
E)6.98 * 10-12
A)4.78 *10-2
B)2.98* 10-3
C)1.19 * 10-3
D)1.31 * 10-3
E)6.98 * 10-12

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
65
What is the percent dissociation of 0.40 M butyric acid (HC4H7O2)? (The Ka value for butyric acid is 1.48 * 10-5.)
A)0.24%
B)0.96%
C)6.1 * 10-3%
D)3.7*10-3%
E)0.61%
A)0.24%
B)0.96%
C)6.1 * 10-3%
D)3.7*10-3%
E)0.61%

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
66
The concentration of acetic acid (pKa = 4.75) in vinegar is about 1.0 M.With this information, what do you predict the pH of vinegar to be?
A)4.75
B)2.38
C)11.62
D)7.00
E)5.35
A)4.75
B)2.38
C)11.62
D)7.00
E)5.35

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
67
What is the pH of a 0.45 M solution of aniline (C6H5NH2)? (pKb =9.40)
A)2.47
B)9.13
C)9.85
D)4.87
E)11.53
A)2.47
B)9.13
C)9.85
D)4.87
E)11.53

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
68
What is the pH of a 0.20 M ammonia solution? (Kb value for ammonia = 1.8 *10-5)
A)11.28
B)9.26
C)4.74
D)9.56
E)2.72
A)11.28
B)9.26
C)4.74
D)9.56
E)2.72

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
69
What is the pH of a 16.0 M HCl solution?
A)1.204
B)(-1.204)
C)0.000
D)12.796
E)15.204
A)1.204
B)(-1.204)
C)0.000
D)12.796
E)15.204

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
70
What is the percent dissociation of 0.20 M iodic acid? (The Ka value for iodic acid, HIO3, is 1.7 *10-1.)
A)59%
B)85%
C)92%
D)12%
E)18%
A)59%
B)85%
C)92%
D)12%
E)18%

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
71
What is the pH of a solution prepared by dissolving 0.025 mol of NaOH in 450.0 mL of solution?
A)1.26
B)12.40
C)4.26
D)9.74
E)12.74
A)1.26
B)12.40
C)4.26
D)9.74
E)12.74

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
72
What is the pH of a 0.010 M acetic acid solution? (Ka for acetic acid = 1.76 *10-5)
A)2.00
B)4.74
C)2.74
D)3.38
E)6.74
A)2.00
B)4.74
C)2.74
D)3.38
E)6.74

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
73
What is the percent dissociation of 0.150 M triethylamine? (The Kb value for triethylamine is 5.25 * 10-4.)
A)5.92%
B)5.75 *10-3%
C)39.4%
D)5.90 *10-3%
E)5.75%
A)5.92%
B)5.75 *10-3%
C)39.4%
D)5.90 *10-3%
E)5.75%

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
74
The first disinfectant used by Joseph Lister was called carbolic acid.This substance now is known as phenol, C6H5OH (pKa = 9.90).What is the pH of a 0.10 M solution of phenol?
A)3.50
B)8.55
C)6.50
D)5.45
E)4.50
A)3.50
B)8.55
C)6.50
D)5.45
E)4.50

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
75
The degree of ionization
A)increases with increasing concentration of a weak acid.
B)decreases with increasing concentration of a weak acid.
C)does not change with changing concentration of a weak acid.
D)is not related to the concentration of a weak acid.
E)is independent of the composition of the weak acid.
A)increases with increasing concentration of a weak acid.
B)decreases with increasing concentration of a weak acid.
C)does not change with changing concentration of a weak acid.
D)is not related to the concentration of a weak acid.
E)is independent of the composition of the weak acid.

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
76
What is the pH of a 0.025 M pyridine solution? (Kb value for pyridine =1.7 *10-9)
A)7.17
B)12.40
C)8.81
D)5.19
E)10.37
A)7.17
B)12.40
C)8.81
D)5.19
E)10.37

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
77
What is the pH of a 0.0250 M HNO3 solution?
A)1.602
B)(-1.602)
C)0.000
D)12.398
E)2.500
A)1.602
B)(-1.602)
C)0.000
D)12.398
E)2.500

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
78
What is the concentration of [OH-] in a 0.20 M ammonia solution? (Kb value for ammonia =1.8 *10-5)
A)3.6 * 10-6 M
B)1.8 *10-5 M
C)0.20 M
D)1.9 *10-3 M
E)4.2 *10-4 M
A)3.6 * 10-6 M
B)1.8 *10-5 M
C)0.20 M
D)1.9 *10-3 M
E)4.2 *10-4 M

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
79
Codeine (C18H21NO3, 299.36 g/mol) is a weak base.Suppose a 2.00 g pain tablet containing 30.0 mg of codeine is dissolved in water to produce a 0.100 L solution with a pH of 9.47.Based on this information, determine the value of Kb for codeine.
A)8.6 *10-6
B)3.4 * 10-9
C)9.0 *10-7
D)2.9 *10-2
E)8.7 *10-7
A)8.6 *10-6
B)3.4 * 10-9
C)9.0 *10-7
D)2.9 *10-2
E)8.7 *10-7

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck
80
The acidic ingredient in vinegar is acetic acid.If the pH of a particular brand of vinegar is 2.40 and the molar concentration of acetic acid in the vinegar is 0.85 M, determine the value of Ka for acetic acid.
A)2.5 * 10-5
B)5.0 * 10-5
C)4.7 * 10-3
D)1.9 *10-5
E)7.4 *10-3
A)2.5 * 10-5
B)5.0 * 10-5
C)4.7 * 10-3
D)1.9 *10-5
E)7.4 *10-3

Unlock Deck
Unlock for access to all 123 flashcards in this deck.
Unlock Deck
k this deck


