Deck 1: Matter and Energy an Atomic Perspective
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/138
Play
Full screen (f)
Deck 1: Matter and Energy an Atomic Perspective
1
A molecule
A)must contain at least two types of atoms.
B)can be an element or a compound.
C)cannot form a solid.
D)cannot be broken into its constituent atoms by any means.
E)can contain only one type of atom.
A)must contain at least two types of atoms.
B)can be an element or a compound.
C)cannot form a solid.
D)cannot be broken into its constituent atoms by any means.
E)can contain only one type of atom.
can be an element or a compound.
2
The law of definite proportions states that
A)compounds such as NO and NO2 have identical chemical properties.
B)compounds such as NO and NO2 must have masses that are whole-number multiples of each other.
C)nitrogen and oxygen can combine to form a variety of compounds, such as NO or NO2.
D)the elements forming a given compound always react in the same proportions.
E)only one compound can be produced when two elements combine.
A)compounds such as NO and NO2 have identical chemical properties.
B)compounds such as NO and NO2 must have masses that are whole-number multiples of each other.
C)nitrogen and oxygen can combine to form a variety of compounds, such as NO or NO2.
D)the elements forming a given compound always react in the same proportions.
E)only one compound can be produced when two elements combine.
the elements forming a given compound always react in the same proportions.
3
An element
A)can be separated into its components by physical methods.
B)has different chemical properties depending on its state.
C)cannot be separated into simpler substances by chemical methods.
D)can also be a compound.
E)exists only as atoms, not as molecules.
A)can be separated into its components by physical methods.
B)has different chemical properties depending on its state.
C)cannot be separated into simpler substances by chemical methods.
D)can also be a compound.
E)exists only as atoms, not as molecules.
cannot be separated into simpler substances by chemical methods.
4
Which of the following does NOT illustrate the law of multiple proportions?
A)The N-to-O mass ratio in NO is 0.875, whereas that in N2O is 1.75.
B)C2H2 has a 12:1 C-to-H mass ratio, while C2H6 has a 4:1 C-to-H mass ratio.
C)The ratio of O:C by mass in CO2 is twice that of CO.
D)If a sample of H2O contains 16 g of oxygen, a sample of H2O2 with the same number of molecules would contain 32 g of oxygen.
E)H2S and H2O contain the same mass of hydrogen.
A)The N-to-O mass ratio in NO is 0.875, whereas that in N2O is 1.75.
B)C2H2 has a 12:1 C-to-H mass ratio, while C2H6 has a 4:1 C-to-H mass ratio.
C)The ratio of O:C by mass in CO2 is twice that of CO.
D)If a sample of H2O contains 16 g of oxygen, a sample of H2O2 with the same number of molecules would contain 32 g of oxygen.
E)H2S and H2O contain the same mass of hydrogen.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
5
For a hypothesis to be considered a valid scientific theory, it must
A)summarize experimental data without trying to predict future results.
B)be impossible to prove wrong by experiment.
C)explain widely observed phenomena based on extensive testing.
D)never be modified or expanded.
E)be voted on by the scientific community and accepted by all.
A)summarize experimental data without trying to predict future results.
B)be impossible to prove wrong by experiment.
C)explain widely observed phenomena based on extensive testing.
D)never be modified or expanded.
E)be voted on by the scientific community and accepted by all.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is NOT an element?
A)Cs
B)Au
C)CS2
D)Ar
E)Co
A)Cs
B)Au
C)CS2
D)Ar
E)Co

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
7
Table sugar (sucrose, C12H22O11) dissolves in water.This process__________
A)is a chemical change.
B)is a physical change.
C)produces a heterogeneous mixture.
D)is a chemical property of sucrose.
E)converts sucrose to carbon dioxide and water.
A)is a chemical change.
B)is a physical change.
C)produces a heterogeneous mixture.
D)is a chemical property of sucrose.
E)converts sucrose to carbon dioxide and water.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
8
A pure substance
A)must be composed of atoms of the same type.
B)cannot be separated into simpler substances by physical means.
C)must be a compound.
D)has different chemical properties depending on its source.
E)can have a composition that varies from sample to sample.
A)must be composed of atoms of the same type.
B)cannot be separated into simpler substances by physical means.
C)must be a compound.
D)has different chemical properties depending on its source.
E)can have a composition that varies from sample to sample.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
9
According to the law of definite proportions,
A)atoms forming a given compound react in variable proportions depending on conditions.
B)different samples of the same compound contain the same proportions of the same elements.
C)all compounds containing the same types of atoms have identical properties.
D)all compounds containing the same types of atoms have relative masses that are whole-number multiples.
E)only one type of molecule can be produced when two elements combine.
A)atoms forming a given compound react in variable proportions depending on conditions.
B)different samples of the same compound contain the same proportions of the same elements.
C)all compounds containing the same types of atoms have identical properties.
D)all compounds containing the same types of atoms have relative masses that are whole-number multiples.
E)only one type of molecule can be produced when two elements combine.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a homogeneous mixture?
A)an egg
B)smoke
C)beach sand
D)dry ice solid CO2
E)a salt solution NaCl dissolved in water)
A)an egg
B)smoke
C)beach sand
D)dry ice solid CO2
E)a salt solution NaCl dissolved in water)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements is NOT true?
A)Given that the chemical formula of methanol is CH4O, the number of carbon atoms in a sample of methanol will be the same as the number of oxygen atoms.
B)If a compound is 75% carbon and 25% hydrogen by mass, 12 g of the compound contains 9 g C and 3 g H.
C)If a compound contains 76 g of chlorine and 12 g of carbon, it will always have a 6.33:1 mass ratio of Cl to C.
D)A compound containing 17.1 g of phosphorus and 58.9 g of chlorine has the same identity as a compound containing 35.7 g P and 204.3 g Cl.
E)A compound containing 106.6 g of copper and 13.4 g of oxygen has the same identity as a compound containing 159.9 g Cu and 20.1 g O.
A)Given that the chemical formula of methanol is CH4O, the number of carbon atoms in a sample of methanol will be the same as the number of oxygen atoms.
B)If a compound is 75% carbon and 25% hydrogen by mass, 12 g of the compound contains 9 g C and 3 g H.
C)If a compound contains 76 g of chlorine and 12 g of carbon, it will always have a 6.33:1 mass ratio of Cl to C.
D)A compound containing 17.1 g of phosphorus and 58.9 g of chlorine has the same identity as a compound containing 35.7 g P and 204.3 g Cl.
E)A compound containing 106.6 g of copper and 13.4 g of oxygen has the same identity as a compound containing 159.9 g Cu and 20.1 g O.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following illustrates the law of multiple proportions?
A)The mass ratio of O to N in NO2 is twice that in NO.
B)NO2 always contains one nitrogen atom and two oxygen atoms.
C)The mass of NO2 is a small whole-number multiple of the mass of NO.
D)NO and NO2 have similar chemical and physical properties.
E)NO2 and N2O4 are the same compound.
A)The mass ratio of O to N in NO2 is twice that in NO.
B)NO2 always contains one nitrogen atom and two oxygen atoms.
C)The mass of NO2 is a small whole-number multiple of the mass of NO.
D)NO and NO2 have similar chemical and physical properties.
E)NO2 and N2O4 are the same compound.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is NOT a pure substance?
A)sparkling water
B)gold metal
C)oxygen gas
D)water vapor
E)dry ice solid (CO2)
A)sparkling water
B)gold metal
C)oxygen gas
D)water vapor
E)dry ice solid (CO2)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following is a hypothesis?
A)Energy is required to vaporize a liquid.
B)The composition of a pure substance is fixed and definite.
C)Hydrogen gas and oxygen gas can react to form water.
D)A Car's battery must be dead because the car won't start.
E)Matter is composed of atoms.
A)Energy is required to vaporize a liquid.
B)The composition of a pure substance is fixed and definite.
C)Hydrogen gas and oxygen gas can react to form water.
D)A Car's battery must be dead because the car won't start.
E)Matter is composed of atoms.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is an element?
A)C6H12O6
B)HNO3
C)NaCl
D)CH4
E)O3
A)C6H12O6
B)HNO3
C)NaCl
D)CH4
E)O3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a pure substance?
A)seawater
B)blood
C)brass an alloy of copper and zinc)
D)table sugarsucrose, C12H22O11.
E)beer
A)seawater
B)blood
C)brass an alloy of copper and zinc)
D)table sugarsucrose, C12H22O11.
E)beer

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is NOT true?
A)The relative numbers of each type of atom in a given compound do not vary.
B)A compound always contains the same mass percentages of its constituent elements.
C)A large sample and a small sample of a given compound contain the same number of each type of atom.
D)A large sample and a small sample of a given compound contain the same types of atoms combined in the same proportions.
E)A large sample and a small sample of a compound share the same chemical formula.
A)The relative numbers of each type of atom in a given compound do not vary.
B)A compound always contains the same mass percentages of its constituent elements.
C)A large sample and a small sample of a given compound contain the same number of each type of atom.
D)A large sample and a small sample of a given compound contain the same types of atoms combined in the same proportions.
E)A large sample and a small sample of a compound share the same chemical formula.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a heterogeneous mixture?
A)concrete
B)sweet tea
C)black coffee
D)mercury metal
E)an intravenous (IV) solution
A)concrete
B)sweet tea
C)black coffee
D)mercury metal
E)an intravenous (IV) solution

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
19
Which step is NOT a part of the scientific method?
A)Form a testable hypothesis.
B)Make observations.
C)Conduct reproducible experiments.
D)Identify different factors that affect results.
E)Stop experimentation once the desired results are achieved.
A)Form a testable hypothesis.
B)Make observations.
C)Conduct reproducible experiments.
D)Identify different factors that affect results.
E)Stop experimentation once the desired results are achieved.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
20
A sample of a compound
A)breaks into its constituent atoms during phase changes.
B)is a homogeneous mixture.
C)contains atoms that can be physically separated from each other.
D)contains at least two types of atoms in a constant, fixed ratio.
E)has a variable composition depending on its temperature.
A)breaks into its constituent atoms during phase changes.
B)is a homogeneous mixture.
C)contains atoms that can be physically separated from each other.
D)contains at least two types of atoms in a constant, fixed ratio.
E)has a variable composition depending on its temperature.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is a chemical property of copper metal?
A)It conducts heat.
B)It reacts with nitric acid to produce copper(II) nitrate.
C)It melts at 1085 C
D)It conducts electricity.
E)It has an orange color.
A)It conducts heat.
B)It reacts with nitric acid to produce copper(II) nitrate.
C)It melts at 1085 C
D)It conducts electricity.
E)It has an orange color.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
22
Which one of the following represents a physical change?
A)Milk turns sour.
B)Rust forms on iron nails.
C)Sugar ferments to form ethanol.
D)An egg begins to smell very bad.
E)Sugar melts and forms a syrupy liquid.
A)Milk turns sour.
B)Rust forms on iron nails.
C)Sugar ferments to form ethanol.
D)An egg begins to smell very bad.
E)Sugar melts and forms a syrupy liquid.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following represents a physical property of water?
A)It boils at 100 C.
B)An electrical current decomposes water into hydrogen gas and oxygen gas.
C)It reacts with iron metal and oxygen to form rust.
D)It reacts with carbon monoxide to form carbon dioxide and hydrogen gas.
E)It is used in photosynthesis.
A)It boils at 100 C.
B)An electrical current decomposes water into hydrogen gas and oxygen gas.
C)It reacts with iron metal and oxygen to form rust.
D)It reacts with carbon monoxide to form carbon dioxide and hydrogen gas.
E)It is used in photosynthesis.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
24
Distillation may be used to separate components in a mixture based on_____
A)solubilities.
B)masses.
C)volatilities.
D)densities.
E)colors.
A)solubilities.
B)masses.
C)volatilities.
D)densities.
E)colors.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a chemical property of acetone C3H6O?
A)It readily evaporates at room temperature.
B)It has a pungent, irritating odor.
C)It can be ignited in oxygen.
D)It boils at 56 C.
E)It is miscible with water.
A)It readily evaporates at room temperature.
B)It has a pungent, irritating odor.
C)It can be ignited in oxygen.
D)It boils at 56 C.
E)It is miscible with water.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
26
Based on values for the volume per gram of the given materials, which of the following would NOT float in water density = 0.997 g/cm3)? 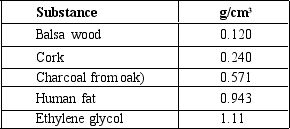
A)balsa wood
B)cork
C)charcoal
D)human fat
E)ethylene glycol

A)balsa wood
B)cork
C)charcoal
D)human fat
E)ethylene glycol

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
27
When copper metal is dropped into nitric acid, a blue solution containing copperII) ions is produced along with brown nitrogen monoxide gas.Which of the following is an example of a chemical property?
A)copper's red-orange appearance
B)nitrogen monoxide's irritating odor
C)the blue color of aqueous copper(II) ions
D)the viscosity of nitric acid at room temperature
E)nitric acid's ability to react with copper metal
A)copper's red-orange appearance
B)nitrogen monoxide's irritating odor
C)the blue color of aqueous copper(II) ions
D)the viscosity of nitric acid at room temperature
E)nitric acid's ability to react with copper metal

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
28
Acetone and water mix to form a homogeneous solution.Acetone has a boiling point of 56 C.Which of the following would be a suitable method for separating acetone from water?
A)filtration
B)combustion
C)distillation
D)scanning tunneling microscopy
E)sublimation
A)filtration
B)combustion
C)distillation
D)scanning tunneling microscopy
E)sublimation

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
29
Which process would be a practical and effective way to separate beta-carotene, an orange pigment, from hexane liquid?
A)filtration
B)chromatography
C)combustion
D)scanning tunneling microscopy
E)sublimation
A)filtration
B)chromatography
C)combustion
D)scanning tunneling microscopy
E)sublimation

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
30
Extensive properties are
A)dependent on the amount of substance present.
B)identical for all substances.
C)independent of a substance's phase.
D)the physical properties of a substance.
E)dependent on the reactivity of the substance.
A)dependent on the amount of substance present.
B)identical for all substances.
C)independent of a substance's phase.
D)the physical properties of a substance.
E)dependent on the reactivity of the substance.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following can be separated by filtration?
A)rust particles in water
B)air dispersed in whipped cream
C)alcohol dissolved in water
D)salt dissolved in water
E)nitrogen from air
A)rust particles in water
B)air dispersed in whipped cream
C)alcohol dissolved in water
D)salt dissolved in water
E)nitrogen from air

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
32
Which represents an extensive property of hydrogen?
A)Hydrogen gas is odorless and colorless.
B)A hydrogen gas molecule is composed of two hydrogen atoms.
C)Hydrogen gas is flammable.
D)Hydrogen releases a given amount of energy when it reacts with oxygen.
E)Hydrogen gas under normal conditions is nonmetallic.
A)Hydrogen gas is odorless and colorless.
B)A hydrogen gas molecule is composed of two hydrogen atoms.
C)Hydrogen gas is flammable.
D)Hydrogen releases a given amount of energy when it reacts with oxygen.
E)Hydrogen gas under normal conditions is nonmetallic.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
33
Which statement is true regarding ammonia, NH3?
A)It can also be correctly represented as N2H6.
B)It cannot be decomposed into simpler substances by any means.
C)Its decomposition produces three volumes of hydrogen for every one volume of nitrogen.
D)It can be separated into nitrogen and hydrogen atoms using distillation.
E)It is not a stable molecule and does not exist at room temperature.
A)It can also be correctly represented as N2H6.
B)It cannot be decomposed into simpler substances by any means.
C)Its decomposition produces three volumes of hydrogen for every one volume of nitrogen.
D)It can be separated into nitrogen and hydrogen atoms using distillation.
E)It is not a stable molecule and does not exist at room temperature.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is an intensive property of chlorine?
A)It has mass.
B)It boils at - 34 - C.
C)Chlorine gas expands to fill a balloon.
D)The reaction of chlorine with hydrogen releases a given amount of energy.
E)Chlorine gas in a container exerts a given pressure at a given temperature.
A)It has mass.
B)It boils at - 34 - C.
C)Chlorine gas expands to fill a balloon.
D)The reaction of chlorine with hydrogen releases a given amount of energy.
E)Chlorine gas in a container exerts a given pressure at a given temperature.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
35
If you had equal masses of each of the following substances, which would occupy the greatest volume?
A)ice (d = 0.917 g/mL)
B)water (d = 0.997 g/mL)
C)beeswax (d = 0.960 g/mL)
D)cocoa butter (d = 0.910 g/mL)
E)aluminum (d = 2.70 g/mL)
A)ice (d = 0.917 g/mL)
B)water (d = 0.997 g/mL)
C)beeswax (d = 0.960 g/mL)
D)cocoa butter (d = 0.910 g/mL)
E)aluminum (d = 2.70 g/mL)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
36
Which one of the following represents a chemical change?
A)Mercury(II) oxide is heated up and forms mercury metal and oxygen gas.
B)Rubbing alcohol evaporates.
C)Iodine vapor deposits on a surface.
D)Iron metal is separated from sand using a magnet.
E)Rock salt is pulverized.
A)Mercury(II) oxide is heated up and forms mercury metal and oxygen gas.
B)Rubbing alcohol evaporates.
C)Iodine vapor deposits on a surface.
D)Iron metal is separated from sand using a magnet.
E)Rock salt is pulverized.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is a chemical property of formaldehyde (CH2O)?
A)It is flammable.
B)It has a density of 1.09 g/mL.
C)It is colorless.
D)It dissolves in water.
E)It is a gas at room temperature.
A)It is flammable.
B)It has a density of 1.09 g/mL.
C)It is colorless.
D)It dissolves in water.
E)It is a gas at room temperature.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following is NOT an intensive physical property of a pure liquid?
A)boiling point
B)conductivity
C)mass
D)density
E)color
A)boiling point
B)conductivity
C)mass
D)density
E)color

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is a chemical property of platinum?
A)It conducts heat and electricity.
B)It can react with chlorine gas to form platinum(IV) chloride.
C)The difference between its melting and boiling points is 2057 C.
D)It is a gray-white metal.
E)Sound travels through it at a speed of 2680 m/s.
A)It conducts heat and electricity.
B)It can react with chlorine gas to form platinum(IV) chloride.
C)The difference between its melting and boiling points is 2057 C.
D)It is a gray-white metal.
E)Sound travels through it at a speed of 2680 m/s.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following represents a chemical property of iron?
A)Its density is 7.84 g/cm3.
B)It is magnetic.
C)It reacts with oxygen in moist air.
D)Its melting point is 1538 C.
E)It conducts electricity.
A)Its density is 7.84 g/cm3.
B)It is magnetic.
C)It reacts with oxygen in moist air.
D)Its melting point is 1538 C.
E)It conducts electricity.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
41
Equal amounts of a pure substance undergo the following changes.Which process would you predict releases the greatest amount of energy?
A)deposition (gas solid)
B)vaporization (liquid gas)
C)freezing (liquid solid)
D)condensation (gas liquid)
E)melting (solid liquid)
A)deposition (gas solid)
B)vaporization (liquid gas)
C)freezing (liquid solid)
D)condensation (gas liquid)
E)melting (solid liquid)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
42
Consider electrons traveling through a copper (Cu) wire at a speed of 0.024 cm/s.What is true about the energy of their motion?
A)It is primarily kinetic.
B)It is primarily potential.
C)It would be unaffected if the speed of the electrons increased.
D)It is strongly affected by gravity.
E)It cannot be used to do work.
A)It is primarily kinetic.
B)It is primarily potential.
C)It would be unaffected if the speed of the electrons increased.
D)It is strongly affected by gravity.
E)It cannot be used to do work.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
43
The chemical formula of dimethyl ether can be represented in different ways.When its formula is written as C2H6O,
A)the arrangement of the atoms in the molecule is evident.
B)only the number and type of atoms of each element are given.
C)its structural formula can be deduced.
D)it shows that no other molecules can have that formula.
E)it lists only one of many possible elemental compositions for dimethyl ether.
A)the arrangement of the atoms in the molecule is evident.
B)only the number and type of atoms of each element are given.
C)its structural formula can be deduced.
D)it shows that no other molecules can have that formula.
E)it lists only one of many possible elemental compositions for dimethyl ether.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements about energy, work, and heat is NOT true?
A)Adding heat to a sample of matter increases the average kinetic energy of its constituent particles.
B)Thermal energy is the portion of the energy of an object that increases as temperature increases.
C)When an object does work, part of the energy it expends is destroyed as it converts to heat.
D)The energy available from some chemical reactions can be used to do work and/or produce heat.
E)Heat involves the transfer of energy from a hotter object to a cooler one.
A)Adding heat to a sample of matter increases the average kinetic energy of its constituent particles.
B)Thermal energy is the portion of the energy of an object that increases as temperature increases.
C)When an object does work, part of the energy it expends is destroyed as it converts to heat.
D)The energy available from some chemical reactions can be used to do work and/or produce heat.
E)Heat involves the transfer of energy from a hotter object to a cooler one.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
45
Work is defined as the exertion of force through a distance.Which of the following is NOT an example of work?
A)Molecules in the air push against the blades of a windmill.
B)Blood is pumped through the circulatory system.
C)Electrons flow against the resistance present in a copper wire.
D)Thermal energy heat) is transferred from a hot stove to the surrounding air.
E)A student lifts a book off of the floor.
A)Molecules in the air push against the blades of a windmill.
B)Blood is pumped through the circulatory system.
C)Electrons flow against the resistance present in a copper wire.
D)Thermal energy heat) is transferred from a hot stove to the surrounding air.
E)A student lifts a book off of the floor.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
46
The space-filling model of a molecule
A)clearly shows bond angles.
B)gives little idea of how atoms are arranged.
C)is best suited for very large molecules.
D)gives an indication of three-dimensional shape.
E)spreads atoms out so they are easy to view.
A)clearly shows bond angles.
B)gives little idea of how atoms are arranged.
C)is best suited for very large molecules.
D)gives an indication of three-dimensional shape.
E)spreads atoms out so they are easy to view.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
47
Equal amounts of water undergo the following changes.Which of the following would involve the largest change in energy?
A)Ice is melted to form liquid water at 0 C.
B)Ice at -25 C is heated to 0 C.
C)Water is heated from 25 C to 50 C.
D)Steam at 100 C is cooled and condensed to form liquid water at 85 C.
E)Water at 0 C is heated and vaporized to form steam at 120 C.
A)Ice is melted to form liquid water at 0 C.
B)Ice at -25 C is heated to 0 C.
C)Water is heated from 25 C to 50 C.
D)Steam at 100 C is cooled and condensed to form liquid water at 85 C.
E)Water at 0 C is heated and vaporized to form steam at 120 C.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
48
Equal amounts of water are present under the following conditions.In which case do the water molecules have the highest kinetic energy?
A)as ice at - 10 - C
B)as steam at 100-C
C)in the liquid phase at 80-C
D)in the gas phase at 150-C
E)in the solid phase at 0-C
A)as ice at - 10 - C
B)as steam at 100-C
C)in the liquid phase at 80-C
D)in the gas phase at 150-C
E)in the solid phase at 0-C

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
49
Which statement correctly describes the properties of gaseous helium He)?
A)The gas is not highly compressible even though the atoms do not occupy the entire volume of the container.
B)The gas is highly compressible because there is a lot of empty space between the atoms.
C)The atoms are moving rapidly about the container, giving the gas its definite shape.
D)The gas has a definite volume and shape because the atoms are not moving about the container.
E)A gas takes the shape of the container, but its total volume cannot change.
A)The gas is not highly compressible even though the atoms do not occupy the entire volume of the container.
B)The gas is highly compressible because there is a lot of empty space between the atoms.
C)The atoms are moving rapidly about the container, giving the gas its definite shape.
D)The gas has a definite volume and shape because the atoms are not moving about the container.
E)A gas takes the shape of the container, but its total volume cannot change.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is the SI base unit for mass?
A)g
B)kg
C)mg
D)lb
E)m
A)g
B)kg
C)mg
D)lb
E)m

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
51
The densities of cork, lead, and water are 0.240 g/cm3, 11.34 g/cm3, and 0.997 g/cm3 at 25 C, respectively.If 20.0 g of lead are placed inside an 85.0 cm3 piece of cork, what is the overall density, and will it float on water?
A)0.466 g/cm3; Yes, it will float.
B)0.235 g/cm3; Yes, it will float.
C)0.211 g/cm3; Yes, it will float.
D)4.25 g/cm3; No, it will not float.
E)2.15 g/cm3; No, it will not float.
A)0.466 g/cm3; Yes, it will float.
B)0.235 g/cm3; Yes, it will float.
C)0.211 g/cm3; Yes, it will float.
D)4.25 g/cm3; No, it will not float.
E)2.15 g/cm3; No, it will not float.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
52
What type of chemical formula is shown for diethyl ether? 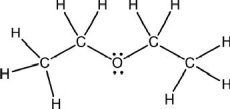
A)molecular
B)structural
C)condensed structural
D)ball-and-stick
E)space-filling

A)molecular
B)structural
C)condensed structural
D)ball-and-stick
E)space-filling

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
53
Which statement describing the properties of the different phases of a sample of matter is true?
A)The particles in both the gas and liquid phases are highly ordered and in close proximity to one another.
B)The particles in the liquid phase are highly compressible because they can slip past one another.
C)The particles in both the solid and liquid phases are free to assume any shape, and their nearest neighbors change over time.
D)The solid phase is rigid, even though its constituent particles may vibrate a little depending on their temperature.
E)Localized areas of order can form in the gas phase because the particles experience significant attractions to one another.
A)The particles in both the gas and liquid phases are highly ordered and in close proximity to one another.
B)The particles in the liquid phase are highly compressible because they can slip past one another.
C)The particles in both the solid and liquid phases are free to assume any shape, and their nearest neighbors change over time.
D)The solid phase is rigid, even though its constituent particles may vibrate a little depending on their temperature.
E)Localized areas of order can form in the gas phase because the particles experience significant attractions to one another.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
54
Ethanol and dimethyl ether molecules both contain two carbon atoms, six hydrogen atoms, and one oxygen atom.Which statement is true?
A)Their molecular formulas are different.
B)They show the same physical properties but different chemical properties.
C)The arrangement of the atoms in each type of molecule is different.
D)Their melting points and boiling points are the same.
E)There is no physical method that can distinguish between the two.
A)Their molecular formulas are different.
B)They show the same physical properties but different chemical properties.
C)The arrangement of the atoms in each type of molecule is different.
D)Their melting points and boiling points are the same.
E)There is no physical method that can distinguish between the two.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
55
Which has the highest kinetic energy, assuming all follow the equation KE - ½ mu2, where m is the mass and u is the velocity?
A)a one-ton (910 kg) truck traveling at 65 miles per hour (29 m/s)
B)an electron with a mass of 9.11 -10-27 kg traveling at 2.97 -108 m/s (99% of the speed of light)
C)an oxygen molecule with a mass of 5.31 -10-26 kg traveling at 394 m/s (roughly its speed at room temperature)
D)Usain Bolt, who has a mass of approximately 94 kg, running at 10 m/s (22.4 miles per hour)
E)an oil tanker with a mass of 3 -107 kg traveling at 9 m/s (20 miles per hour)
A)a one-ton (910 kg) truck traveling at 65 miles per hour (29 m/s)
B)an electron with a mass of 9.11 -10-27 kg traveling at 2.97 -108 m/s (99% of the speed of light)
C)an oxygen molecule with a mass of 5.31 -10-26 kg traveling at 394 m/s (roughly its speed at room temperature)
D)Usain Bolt, who has a mass of approximately 94 kg, running at 10 m/s (22.4 miles per hour)
E)an oil tanker with a mass of 3 -107 kg traveling at 9 m/s (20 miles per hour)

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
56
If the speed of an object triples, its kinetic energy
A)increases by a factor of 3.
B)increases by a factor of 9.
C)decreases by a factor of 3.
D)decreases by a factor of 9.
E)is unaffected.
A)increases by a factor of 3.
B)increases by a factor of 9.
C)decreases by a factor of 3.
D)decreases by a factor of 9.
E)is unaffected.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
57
Solid carbon dioxide CO2 can undergo sublimation to form gaseous CO2.Which of the following statements is true?
A)In the solid phase, CO2 molecules easily slip past each other, and there are areas of randomly ordered molecules.
B)In the gas phase, CO2 molecules are strongly attracted to each other.
C)The motion of the CO2 molecules in the solid phase is much more restricted than in the gas phase.
D)CO2 molecules in the solid phase are easily compressed to smaller volumes.
E)The CO2 molecules decompose to form carbon and oxygen when they enter the gas phase.
A)In the solid phase, CO2 molecules easily slip past each other, and there are areas of randomly ordered molecules.
B)In the gas phase, CO2 molecules are strongly attracted to each other.
C)The motion of the CO2 molecules in the solid phase is much more restricted than in the gas phase.
D)CO2 molecules in the solid phase are easily compressed to smaller volumes.
E)The CO2 molecules decompose to form carbon and oxygen when they enter the gas phase.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
58
Soft solder is a blended alloy of tin 7.31 g/cm3) and lead 11.34 g/cm3) that is used in plumbing and electronics.It is 63.5% tin by mass.What is the density of the alloy?
A)9.87 g/cm3
B)8.27 g/cm3
C)7.83 g/cm3
D)8.79 g/cm3
E)9.33 g/cm3
A)9.87 g/cm3
B)8.27 g/cm3
C)7.83 g/cm3
D)8.79 g/cm3
E)9.33 g/cm3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is an example of potential energy?
A)water running down a hill
B)chemical bonds in table sugar sucrose
C)electrons flowing through a wire
D)a crowd moving a barricade
E)molecules moving randomly in a liquid
A)water running down a hill
B)chemical bonds in table sugar sucrose
C)electrons flowing through a wire
D)a crowd moving a barricade
E)molecules moving randomly in a liquid

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
60
At what velocity would a proton be traveling if it had the same kinetic energy as an electron traveling at 10.0% of the speed of light? KE = ½ mu2, where m is the mass and u is the velocity; proton mass = 1.673 *10-27 kg; electron mass = 9.109 * 10-31 kg; speed of light = 2.998 -108 m/s.
A)4.89 *1011 m/s
B)2.45 * 1011 m/s
C)4.95 * 105 m/s
D)7.00 * 105 m/s
E)3.50 *105 m/s
A)4.89 *1011 m/s
B)2.45 * 1011 m/s
C)4.95 * 105 m/s
D)7.00 * 105 m/s
E)3.50 *105 m/s

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
61
If the following arithmetic operations are carried out, how many significant figures should be reported in the answer? 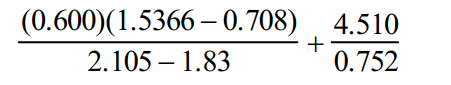
A)1
B)2
C)3
D)4
E)5
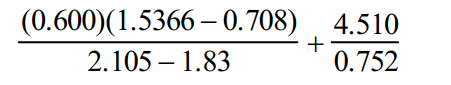
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
62
The average volume of a red blood cell is approximately 90 fL.Express the average value in liters using correct exponential notation and number of significiant figures.
A)90 *10-15 L
B)9 *10-15 L
C)9.0 * 10-15 L
D)9 * 10-14 L
E)9.0 * 10-14 L
A)90 *10-15 L
B)9 *10-15 L
C)9.0 * 10-15 L
D)9 * 10-14 L
E)9.0 * 10-14 L

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
63
The distance between the two hydrogen atoms in a water molecule is about 1.355 *10-10 m.This is equal to _______.
A)1.355 *10-8 mm.
B)1.355 *106 cm.
C)1.355 * 10-6 m.
D)13.55 nm.
E)135.5 pm.
A)1.355 *10-8 mm.
B)1.355 *106 cm.
C)1.355 * 10-6 m.
D)13.55 nm.
E)135.5 pm.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
64
Green light in the visible portion of the electromagnetic radiation spectrum has wavelengths around 550 nm.Express this wavelength in meters using exponential notation.
A)5.5 * 10-9 m
B)5.5 * 10-7 m
C)5.5 m
D)5.5 * 107 m
E)5.5 * 109 m
A)5.5 * 10-9 m
B)5.5 * 10-7 m
C)5.5 m
D)5.5 * 107 m
E)5.5 * 109 m

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
65
A student performed three measurements to determine the density of water at 25 C to four significant figures.The known density of water at 25 C to three significant figures is 0.997 g/mL.The student obtained the following results.  The measurements were _______.
The measurements were _______.
A)sufficiently precise but not accurate.
B)sufficiently accurate but not precise.
C)both sufficiently precise and accurate.
D)neither sufficiently precise nor accurate.
E)not repeated an adequate number of times.
 The measurements were _______.
The measurements were _______.A)sufficiently precise but not accurate.
B)sufficiently accurate but not precise.
C)both sufficiently precise and accurate.
D)neither sufficiently precise nor accurate.
E)not repeated an adequate number of times.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
66
The average diameter of a red blood cell is about 7 *10-6 m.Choose the best way to represent this distance using SI units and prefixes.
A)7 µm
B)0.007 mm
C)0.000007 m
D)7000 nm
E)7000000 pm
A)7 µm
B)0.007 mm
C)0.000007 m
D)7000 nm
E)7000000 pm

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
67
If the following arithmetic operations are carried out, how many significant figures should be reported in the answer?
32 +0.56 + 0.210+ 3.3
A)1
B)2
C)3
D)4
E)5
32 +0.56 + 0.210+ 3.3
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
68
A graduated cylinder is filled with water to the 25.0 mL mark.After 27.5 g of titanium dioxide TiO2 is added, the volume is 31.5 mL.Calculate the density of TiO2.
A)0.873 g/cm3
B)0.87 g/cm3
C)4.2 g/cm3
D)4.23 g/cm3
E)2.05 g/cm3
A)0.873 g/cm3
B)0.87 g/cm3
C)4.2 g/cm3
D)4.23 g/cm3
E)2.05 g/cm3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is not an SI base unit?
A)( C)
B)s
C)kg
D)mol
E)m
A)( C)
B)s
C)kg
D)mol
E)m

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following common laboratory devices will deliver 25 mL of a solution with the greatest precision?
A)a 100 mL beaker without volume divisions
B)a 50 mL beaker with volume divisions every 10 mL
C)a 50 mL graduated cylinder with volume divisions every 2 mL
D)a 25 mL beaker without volume divisions
E)a 25 mL pipet with a to-deliver error of 0.01 mL
A)a 100 mL beaker without volume divisions
B)a 50 mL beaker with volume divisions every 10 mL
C)a 50 mL graduated cylinder with volume divisions every 2 mL
D)a 25 mL beaker without volume divisions
E)a 25 mL pipet with a to-deliver error of 0.01 mL

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following does not show a correct relationship between units?
A)1*103 g = 1 kg
B)1 * 10-3 s = 1 ms
C)1 nm = 1 * 10-9 m
D)1 GB = 1 * 109 B
E)1 * 10-6 µL = 1 L
A)1*103 g = 1 kg
B)1 * 10-3 s = 1 ms
C)1 nm = 1 * 10-9 m
D)1 GB = 1 * 109 B
E)1 * 10-6 µL = 1 L

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
72
Based on the following figure, which of the measurements listed is the best estimate of the length of the object? 
A)1.8 cm
B)1.81 cm
C)1.810 cm
D)1.90 cm
E)1.9 cm

A)1.8 cm
B)1.81 cm
C)1.810 cm
D)1.90 cm
E)1.9 cm

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
73
What value should be reported for the total mass of three samples of iron weighing 117.0 g, 19.43 g, and 6.1043 g?
A)143 g
B)142.53 g
C)142.534 g
D)142.5 g
E)142.5343 g
A)143 g
B)142.53 g
C)142.534 g
D)142.5 g
E)142.5343 g

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
74
An 84.6419 g antique coin is thought to be gold.When the coin is placed in a graduated cylinder containing 15.53 mL of water, the water level rises to 24.64 mL.Calculate the density of the coin.
A)9.29 g/mL
B)5.450 g/mL
C)0.73833 g/mL
D)9.2911 g/mL
E)3.435 g/mL
A)9.29 g/mL
B)5.450 g/mL
C)0.73833 g/mL
D)9.2911 g/mL
E)3.435 g/mL

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following represents the smallest mass?
A)4.0 *100 mg
B)4.0 *102 ng
C)4.0 *10-4 g
D)4.0* 102 F g
E)4.0*10-6 kg
A)4.0 *100 mg
B)4.0 *102 ng
C)4.0 *10-4 g
D)4.0* 102 F g
E)4.0*10-6 kg

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
76
The calculated diameter of a carbon atom is about 0.000000000340 m.In correct scientific notation, this is equal to _______.
A)3.40 * 10-12 km.
B)3.40 * 10-12 cm.
C)3.40 * 10-8 mm.
D)3.40 * 100 nm.
E)3.40 *102 pm.
A)3.40 * 10-12 km.
B)3.40 * 10-12 cm.
C)3.40 * 10-8 mm.
D)3.40 * 100 nm.
E)3.40 *102 pm.

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
77
White fuming nitric acid should contain no more than 2% water by mass.The water content in four samples was measured.What is the average value, and which measured value is closest to the average? 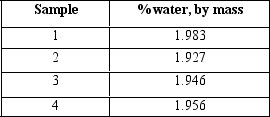
A)1.953, sample 4
B)1.95, sample 4
C)1.9530, sample 4
D)1.9530, sample 3
E)1.953, sample 3

A)1.953, sample 4
B)1.95, sample 4
C)1.9530, sample 4
D)1.9530, sample 3
E)1.953, sample 3

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
78
A rectangular sheet of aluminum foil has a length of 8.0 cm, a width of 4.0 cm, and a mass of 864 mg.Determine the thickness of the foil, given that the density of aluminum is 2.70 g/cm3.
A)1.0 mm
B)0.10 mm
C)0.010 mm
D)10. m
E)1.0 *102 cm
A)1.0 mm
B)0.10 mm
C)0.010 mm
D)10. m
E)1.0 *102 cm

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following does NOT show an exact relationship?
A)100 cm = 1 m
B)1 m3 = 1000 L
C)1 in = 2.54 cm
D)1 km = 0.6214 mi
E)1 dozen = 12 objects
A)100 cm = 1 m
B)1 m3 = 1000 L
C)1 in = 2.54 cm
D)1 km = 0.6214 mi
E)1 dozen = 12 objects

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following represents the largest mass?
A)250 ng
B)25 µg
C)2.5 g
D)0.25 kg
E)25 mg
A)250 ng
B)25 µg
C)2.5 g
D)0.25 kg
E)25 mg

Unlock Deck
Unlock for access to all 138 flashcards in this deck.
Unlock Deck
k this deck


