Deck 2: Chemical Principles
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 2: Chemical Principles
1
Table 2.2 NaOH ⇌ Na+ + OH- base HF ⇌ H+ + F- - acid
MgSO4 ⇌ Mg2+ + SO42- salt KH2PO4 ⇌ K+ + H2PO4- acid H2SO4 ⇌ 2H+ + SO42- salt
Which of the following statements about the reactions in Table 2.2 is FALSE?
A) They are exchange reactions.
B) They are dissociation reactions.
C) They are ionization reactions.
D) They occur when the reactants are dissolved in water.
E) They are reversible reactions.
MgSO4 ⇌ Mg2+ + SO42- salt KH2PO4 ⇌ K+ + H2PO4- acid H2SO4 ⇌ 2H+ + SO42- salt
Which of the following statements about the reactions in Table 2.2 is FALSE?
A) They are exchange reactions.
B) They are dissociation reactions.
C) They are ionization reactions.
D) They occur when the reactants are dissolved in water.
E) They are reversible reactions.
A
2
Which type of molecule contains the alcohol glycerol?
A) protein
B) phospholipids
C) carbohydrate
D) DNA
A) protein
B) phospholipids
C) carbohydrate
D) DNA
B
3
Identify the following reaction: Lactose + H2O → Glucose + Galactose
A) dehydration synthesis reaction
B) hydrolysis reaction
C) exchange reaction
D) reversible reaction
E) ionic reaction
A) dehydration synthesis reaction
B) hydrolysis reaction
C) exchange reaction
D) reversible reaction
E) ionic reaction
B
4
Which of the following statements is FALSE?
A) Water freezes from the top down.
B) Water is formed as a part of a dehydration synthesis reaction.
C) Water molecules are formed by hydrolysis.
D) Water is a polar molecule.
E) Salts readily dissolve in water.
A) Water freezes from the top down.
B) Water is formed as a part of a dehydration synthesis reaction.
C) Water molecules are formed by hydrolysis.
D) Water is a polar molecule.
E) Salts readily dissolve in water.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the following reaction: NH4OH ⇌ NH3 + H2O
A) dehydration synthesis reaction
B) hydrolysis reaction
C) exchange reaction
D) reversible reaction
E) ionic reaction
A) dehydration synthesis reaction
B) hydrolysis reaction
C) exchange reaction
D) reversible reaction
E) ionic reaction

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
Antacids neutralize acid by the following reaction. Identify the salt in the following equation: Mg(OH)2 + 2HCl → MgCl2 + H2O
A) Mg(OH)2
B) HCl
C) H2O
D) MgCl2
E) None of the answers is correct.
A) Mg(OH)2
B) HCl
C) H2O
D) MgCl2
E) None of the answers is correct.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the type of bond between molecules of water in a beaker of water?
A) ionic bond
B) covalent bond
C) hydrogen bond
A) ionic bond
B) covalent bond
C) hydrogen bond

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
Which type of molecule NEVER contains a phosphate group?
A) nucleic acid
B) phospholipid
C) triglycerides
D) ATP
A) nucleic acid
B) phospholipid
C) triglycerides
D) ATP

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
Identify the following reaction: Glucose + Fructose → Sucrose + Water
A) dehydration synthesis reaction
B) hydrolysis reaction
C) exchange reaction
D) reversible reaction
E) ionic reaction
A) dehydration synthesis reaction
B) hydrolysis reaction
C) exchange reaction
D) reversible reaction
E) ionic reaction

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
Table 2.1 16 O 12 C 1 H
8 6 1
Using the information in Table 2.1, calculate the number of moles in 92 grams of ethanol, C2H5OH.
A) 1
B) 2
C) 3
D) 4
E) The answer cannot be determined.
8 6 1
Using the information in Table 2.1, calculate the number of moles in 92 grams of ethanol, C2H5OH.
A) 1
B) 2
C) 3
D) 4
E) The answer cannot be determined.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
Based upon the valence numbers of the elements magnesium (2) and hydrogen (1), predict how many covalent bonds would form between these atoms to achieve the full complement of electrons in their outermost energy shells.
A) one
B) two
C) three
D) four
A) one
B) two
C) three
D) four

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements about the atom 12 C is FALSE? 6
A) It has 12 neutrons in its nucleus.
B) It has 6 electrons orbiting the nucleus.
C) It has 6 protons in its nucleus.
D) Its atomic weight is 12.
E) Its atomic number is 6.
A) It has 12 neutrons in its nucleus.
B) It has 6 electrons orbiting the nucleus.
C) It has 6 protons in its nucleus.
D) Its atomic weight is 12.
E) Its atomic number is 6.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
Which type of molecule is composed of (CH2O) units?
A) nucleic acid
B) lipid
C) carbohydrate
D) protein
A) nucleic acid
B) lipid
C) carbohydrate
D) protein

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
What is the type of bond holding hydrogen and oxygen atoms together in a single H 2O molecule?
A) ionic bond
B) covalent bond
C) hydrogen bond
A) ionic bond
B) covalent bond
C) hydrogen bond

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
Table 2.1 16 O 12 C 1 H
8 6 1
Using the information in Table 2.1, calculate the molecular weight of ethanol, C 2H5OH.
A) 46
B) 34
C) 96
D) 33
E) The answer cannot be determined.
8 6 1
Using the information in Table 2.1, calculate the molecular weight of ethanol, C 2H5OH.
A) 46
B) 34
C) 96
D) 33
E) The answer cannot be determined.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
Which type of molecule contains -NH2 (amino) groups?
A) carbohydrate
B) triglycerides
C) protein
D) nucleic acid
A) carbohydrate
B) triglycerides
C) protein
D) nucleic acid

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements regarding protein structure is FALSE?
A) Quaternary structures involved multiple polypeptides.
B) Tertiary structures are formed only from covalent bonds.
C) The primary structure is formed by covalent bonding between amino acid subunits.
D) Secondary structures are formed only from hydrogen bonds.
A) Quaternary structures involved multiple polypeptides.
B) Tertiary structures are formed only from covalent bonds.
C) The primary structure is formed by covalent bonding between amino acid subunits.
D) Secondary structures are formed only from hydrogen bonds.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following pairs is mismatched?
A) MgSO4 ⇌ Mg2+ + SO42- salt
B) KH2PO4 ⇌ K+ + H2PO4- acid
C) HF ⇌ H+ + F- ‐ acid
D) H2SO4 ⇌ 2H+ + SO42- acid
E) NaOH ⇌ Na+ + OH- base
A) MgSO4 ⇌ Mg2+ + SO42- salt
B) KH2PO4 ⇌ K+ + H2PO4- acid
C) HF ⇌ H+ + F- ‐ acid
D) H2SO4 ⇌ 2H+ + SO42- acid
E) NaOH ⇌ Na+ + OH- base

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the type of bond holding K+ and I- ions in KI?
A) ionic bond
B) covalent bond
C) hydrogen bond
A) ionic bond
B) covalent bond
C) hydrogen bond

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the following reaction: HCl + NaHCO3 → NaCl + H2CO3
A) dehydration synthesis reaction
B) hydrolysis reaction
C) exchange reaction
D) reversible reaction
E) ionic reaction
A) dehydration synthesis reaction
B) hydrolysis reaction
C) exchange reaction
D) reversible reaction
E) ionic reaction

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
Figure 2.3 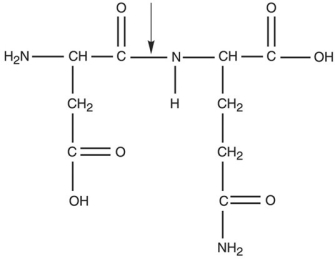 What kind of bond is at the arrow in Figure 2.3?
What kind of bond is at the arrow in Figure 2.3?
A) ionic bond
B) hydrogen bond
C) double covalent bond
D) peptide bond
E) disulfide bridge
 What kind of bond is at the arrow in Figure 2.3?
What kind of bond is at the arrow in Figure 2.3?A) ionic bond
B) hydrogen bond
C) double covalent bond
D) peptide bond
E) disulfide bridge

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
An E. coli culture that has been growing at 37°C is moved to 25°C. Which of the following changes must be made in its plasma membrane to help it cope with the decrease in temperature?
A) The number of saturated chains must increase.
B) The viscosity must increase.
C) The number of unsaturated chains must increase.
D) The number of phosphate groups must increase.
E) No changes are necessary.
A) The number of saturated chains must increase.
B) The viscosity must increase.
C) The number of unsaturated chains must increase.
D) The number of phosphate groups must increase.
E) No changes are necessary.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
Figure 2.1
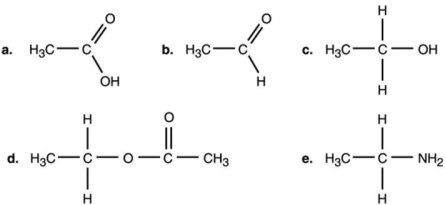
Which compound in Figure 2.1 is an ester?
A) a
B) b
C) c
D) d
E) e

Which compound in Figure 2.1 is an ester?
A) a
B) b
C) c
D) d
E) e

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
Most amino acids found in cells demonstrate what type of chirality?
A) A-isomers
B) L-isomers
C) D-isomers
D) C-isomers
E) B-isomers
A) A-isomers
B) L-isomers
C) D-isomers
D) C-isomers
E) B-isomers

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
Two glucose molecules are combined to make a maltose molecule. What is the chemical formula for maltose?
A) C3H6O3
B) C6H12O6
C) C12H24O12
D) C12H23O10
E) C12H22O11
A) C3H6O3
B) C6H12O6
C) C12H24O12
D) C12H23O10
E) C12H22O11

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
Radioisotopes are frequently used to label molecules in a cell. The fate of atoms and molecules in a cell can then be followed. Assume Saccharomyces cerevisiae is grown in a nutrient medium containing the radioisotope 32P. After a 48-hour incubation, the majority of the 32P would be found in the S. cerevisiaeʹs
A) cell wall.
B) water.
C) proteins.
D) plasma membrane.
E) carbohydrates.
A) cell wall.
B) water.
C) proteins.
D) plasma membrane.
E) carbohydrates.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
What is the type of bond between ions in salt?
A) hydrogen bond
B) covalent bond
C) ionic bond
A) hydrogen bond
B) covalent bond
C) ionic bond

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
Figure 2.1

Which compound in Figure 2.1 is an organic acid?
A) a
B) b
C) c
D) d
E) e

Which compound in Figure 2.1 is an organic acid?
A) a
B) b
C) c
D) d
E) e

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
Which molecule is composed of a chain of amino acids?
A) carbohydrate
B) lipid
C) protein
D) nucleic acid
A) carbohydrate
B) lipid
C) protein
D) nucleic acid

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
What is the type of strong chemical bond between carbon, hydrogen, and oxygen atoms in a single organic molecule?
A) hydrogen bond
B) ionic bond
C) covalent bond
A) hydrogen bond
B) ionic bond
C) covalent bond

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
Radioisotopes are frequently used to label molecules in a cell. The fate of atoms and molecules in a cell can then be followed. Assume Saccharomyces cerevisiae is grown in a nutrient medium containing the radioisotope 35S. After a 48-hour incubation, the 35S would most likely be found in the S. cerevisiaeʹs
A) proteins.
B) nucleic acids.
C) lipids.
D) carbohydrates.
E) water.
A) proteins.
B) nucleic acids.
C) lipids.
D) carbohydrates.
E) water.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
The antimicrobial drug imidazole inhibits sterol synthesis. This would most likely interfere with
A) bacterial cell walls.
B) prokaryotic plasma membranes.
C) fungal cell walls.
D) genes.
E) eukaryotic plasma membranes.
A) bacterial cell walls.
B) prokaryotic plasma membranes.
C) fungal cell walls.
D) genes.
E) eukaryotic plasma membranes.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
Figure 2.1

In Figure 2.1, which is an alcohol?
A) a
B) b
C) c
D) d
E) e

In Figure 2.1, which is an alcohol?
A) a
B) b
C) c
D) d
E) e

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
What is the type of weak bond between the hydrogen of one molecule and the nitrogen of another molecule, where the two donʹt actively share an electron?
A) hydrogen bond
B) hydrophobic bond
C) disulfide bond
D) covalent bond
E) ionic bond
A) hydrogen bond
B) hydrophobic bond
C) disulfide bond
D) covalent bond
E) ionic bond

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
Structurally, ATP is most like which type of molecule?
A) carbohydrate
B) lipid
C) protein
D) nucleic acid
A) carbohydrate
B) lipid
C) protein
D) nucleic acid

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
A scientist wants to perform a test that will indicate whether a nucleic acid sample is composed of RNA or DNA. Testing for the presence of which of the following is most appropriate in this situation?
A) thymine
B) guanine
C) uracil
D) nitrogen
E) phosphate
A) thymine
B) guanine
C) uracil
D) nitrogen
E) phosphate

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
Which are the primary molecules making up plasma membranes in cells?
A) carbohydrates
B) lipids
C) proteins
D) nucleic acids
A) carbohydrates
B) lipids
C) proteins
D) nucleic acids

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
What do genes consist of?
A) carbohydrates
B) lipids
C) proteins
D) nucleic acids
A) carbohydrates
B) lipids
C) proteins
D) nucleic acids

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
Starch, dextran, glycogen, and cellulose are polymers of
A) amino acids.
B) fatty acids.
C) glucose.
D) acids.
E) nucleic acids.
A) amino acids.
B) fatty acids.
C) glucose.
D) acids.
E) nucleic acids.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a base?
A) H2CO
B) C2H5OCOOH → H+ + C2H5OCOO-
C) NaOH → Na+ + OH-
D) H2O → H+ + OH-
E) C2H5OH
A) H2CO
B) C2H5OCOOH → H+ + C2H5OCOO-
C) NaOH → Na+ + OH-
D) H2O → H+ + OH-
E) C2H5OH

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
Elements only achieve the full complement of electrons in outermost energy cells by donating away or sharing electrons.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
All chemical reactions are, in theory, reversible.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
Covalent bonds are always shared equally.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
All forms of life function optimally at a pH of 7.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
There are some forms of life on Earth that can survive without water.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
If an amino acid contained a hydrocarbon (a group of multiple carbons and hydrogens linked together) as its side group, in which of the following categories could it be appropriately designated?
A) polar
B) nonpolar
C) basic
D) acidic
E) hydrophilic
A) polar
B) nonpolar
C) basic
D) acidic
E) hydrophilic

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
Describe how the properties of phospholipids make these molecules well suited for plasma membranes.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
Individual covalent bonds are stronger than individual ionic bonds.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
A basic solution is expected to contain more hydrogen ions than hydroxyl ions.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
Any compound that contains carbon is considered to be strictly organic.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
The density of liquid water is greater than the density of ice.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
The formation of ADP from ATP can be defined as a hydrolytic reaction.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


