Deck 3: Light and Matter: the Inner Workings of the Cosmos
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/112
Play
Full screen (f)
Deck 3: Light and Matter: the Inner Workings of the Cosmos
1
Wave energy can only be transmitted through a material medium.
False
2
The spectral lines of each element are distinctive to that element, whether we are looking at emission or absorption lines.
True
3
Observations in the X- ray portion of the spectrum are routinely done from the surface of the Earth.
False
4
According to Wein's law, the larger the blackbody, the shorter its peak wavelength.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
5
The shorter a wave's wavelength, the greater its energy.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
6
Radio waves, visible light, and X- rays are all types of electromagnetic radiation.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
7
The greater the disturbance of the medium, the higher the amplitude of the wave.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
8
As white light passes through a prism, the red (longer) wavelengths bend less than the blue (shorter) wavelengths, so forming the rainbow of colors.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
9
Doubling the temperature of a blackbody will double the total energy it radiates.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
10
A blue star has a higher surface temperature than a red star.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
11
If a new wave arrives on shore every two seconds, then its frequency is 2 Hz.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
12
An emission line results from an electron falling from a higher to lower energy orbital around its atomic nucleus.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
13
Changing the electric field will have no effect on the magnetic fields of a body.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
14
According to Wein's law, the higher the surface temperature of a star, the redder its color.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
15
As they move through space, the vibrating electrical and magnetic fields of a light wave must move perpendicular to each other.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
16
An absorption line spectrum, with dark lines crossing the rainbow of the continuum, is produced by a low- density hot gas.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
17
While gravity is always attractive, electromagnetic forces are always repulsive.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
18
The frequency of a water wave gives us its height.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
19
In blackbody radiation, the energy is radiated uniformly in every region of the spectrum, so the radiating body appears black in color.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
20
As a star's temperature increases, the frequency of peak emission also increases.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
21
When an electron in a hydrogen atom drops from the second to the first excited energy state it emits a bright red emission line called hydrogen alpha.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
22
Which of these is the same for all forms of electromagnetic (E- M) radiation in a vacuum?
A) frequency
B) wavelength
C) speed
D) amplitude
E) photon energy
A) frequency
B) wavelength
C) speed
D) amplitude
E) photon energy

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
23
The speed of light in a vacuum is
A) 186,000 miles per hour.
B) h = E/c.
C) 300,000 km/sec.
D) 768 km/hour.
E) none of the above.
A) 186,000 miles per hour.
B) h = E/c.
C) 300,000 km/sec.
D) 768 km/hour.
E) none of the above.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
24
The broader the spectral line, the higher the pressure of the gas that is creating it.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
25
The Doppler effect can reveal the rotation speed of a star by the splitting of the spectral lines.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
26
The larger the redshift, the faster the distant galaxy is rushing toward us.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
27
In the Doppler effect, a redshift of spectral lines shows us the source is receding from us.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
28
Spectral lines are produced when an electron makes a transition from one energy state to another.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
29
Medium A blocks more of a certain wavelength of radiation than medium B. Medium A has a higher
A) opacity.
B) transparency.
C) clarity.
D) seeing.
E) albedo.
A) opacity.
B) transparency.
C) clarity.
D) seeing.
E) albedo.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
30
You would perceive a change in a visible light wave's amplitude as a change in its color.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
31
If a fire truck's siren is rising in pitch, it must be approaching us.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
32
The two forms of electromagnetic (E- M) radiation that experience the least atmospheric opacity are
A) microwaves and radio waves.
B) X and gamma radiation.
C) ultraviolet and infrared waves.
D) visible light and infrared waves.
E) visible light and radio waves.
A) microwaves and radio waves.
B) X and gamma radiation.
C) ultraviolet and infrared waves.
D) visible light and infrared waves.
E) visible light and radio waves.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
33
In the Bohr model of the atom, an electron can only exist in specific, well- defined energy levels.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
34
A wave's velocity is the product of the
A) frequency times the wavelength of the wave.
B) frequency times the period of the wave.
C) period times the energy of the wave.
D) amplitude times the frequency of the wave.
E) amplitude times the wavelength of the wave.
A) frequency times the wavelength of the wave.
B) frequency times the period of the wave.
C) period times the energy of the wave.
D) amplitude times the frequency of the wave.
E) amplitude times the wavelength of the wave.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
35
Spectroscopy of a star can reveal its temperature, composition, and line- of- sight motion.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
36
The Zeeman effect reveals the presence of strong magnetic fields by the splitting of spectral lines.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
37
If a wave's frequency doubles and its speed stays constant, its wavelength
A) is now 4× longer.
B) is halved.
C) is also doubled.
D) becomes 16× longer.
E) is unchanged, as c is constant.
A) is now 4× longer.
B) is halved.
C) is also doubled.
D) becomes 16× longer.
E) is unchanged, as c is constant.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
38
Which of these is NOT a form of electromagnetic radiation?
A) X- rays in the doctor's office
B) DC current from your car battery
C) ultraviolet causing a suntan
D) light from your camp fire
E) radio signals
A) X- rays in the doctor's office
B) DC current from your car battery
C) ultraviolet causing a suntan
D) light from your camp fire
E) radio signals

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
39
The radiation our eyes are most sensitive to is the color
A) black at 227 nm.
B) violet at 7,000 Angstroms.
C) red at 6563 Angstroms.
D) yellow- green at about 550 nm.
E) blue at 4,321 nm.
A) black at 227 nm.
B) violet at 7,000 Angstroms.
C) red at 6563 Angstroms.
D) yellow- green at about 550 nm.
E) blue at 4,321 nm.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
40
Consider this diagram. Which statement is true? 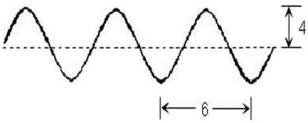
A) The amplitude is 8 and the wavelength is 6.
B) The amplitude is 8 and the wavelength is 12.
C) The amplitude is 6 and the wavelength is 4.
D) The amplitude is 4 and the wavelength is 12.
E) The amplitude is 4 and the wavelength is 6.

A) The amplitude is 8 and the wavelength is 6.
B) The amplitude is 8 and the wavelength is 12.
C) The amplitude is 6 and the wavelength is 4.
D) The amplitude is 4 and the wavelength is 12.
E) The amplitude is 4 and the wavelength is 6.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
41
The element first found in the Sun's spectrum, then on Earth 30 years later, is
A) technicum.
B) hydrogen.
C) helium.
D) aluminum.
E) solarium.
A) technicum.
B) hydrogen.
C) helium.
D) aluminum.
E) solarium.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
42
Which of these is emitted when an electron falls from a higher to lower orbital?
A) a neutrino
B) a positron
C) a graviton
D) a photon
E) another electron
A) a neutrino
B) a positron
C) a graviton
D) a photon
E) another electron

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
43
In Bohr's model of the atom, electrons
A) can be halfway between orbits.
B) are not confined to specific orbits.
C) are spread uniformly through a large, positive mass.
D) move from one orbit to the next orbit in many small steps.
E) only make transitions between orbits of specific energies.
A) can be halfway between orbits.
B) are not confined to specific orbits.
C) are spread uniformly through a large, positive mass.
D) move from one orbit to the next orbit in many small steps.
E) only make transitions between orbits of specific energies.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
44
What is the name of the temperature scale that places zero at the point where all atomic and molecular motion ceases?
A) Ransom
B) Fahrenheit
C) Kelvin
D) Centigrade
E) Celsius
A) Ransom
B) Fahrenheit
C) Kelvin
D) Centigrade
E) Celsius

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
45
For hydrogen, the transition from the first to third excited state produces
A) a blue green absorption line.
B) an ultraviolet line.
C) a red emission line.
D) an infrared line.
E) a violet emission line.
A) a blue green absorption line.
B) an ultraviolet line.
C) a red emission line.
D) an infrared line.
E) a violet emission line.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
46
The Sun's observed spectrum is
A) a continuum with absorption lines.
B) only absorption lines on a black background.
C) a continuum with no lines, as shown by the rainbow.
D) a continuum with emission lines.
E) only emission lines on a black background.
A) a continuum with absorption lines.
B) only absorption lines on a black background.
C) a continuum with no lines, as shown by the rainbow.
D) a continuum with emission lines.
E) only emission lines on a black background.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
47
A jar filled with gas is placed directly in front of a second jar filled with gas. Using a spectroscope to look at one jar through the other you observe dark spectral lines. The jar closest to you contains
A) gas at the same temperature as the other jar.
B) the cooler gas.
C) gas at very high pressure.
D) the hotter gas.
E) the exact same gas as the other jar.
A) gas at the same temperature as the other jar.
B) the cooler gas.
C) gas at very high pressure.
D) the hotter gas.
E) the exact same gas as the other jar.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
48
In a hydrogen atom, a transition from the 2nd to the 1st excited state will produce
A) the bright red Balmer alpha emission line.
B) three different emission lines.
C) a dark absorption line.
D) an ultraviolet spectral line.
E) no emission line.
A) the bright red Balmer alpha emission line.
B) three different emission lines.
C) a dark absorption line.
D) an ultraviolet spectral line.
E) no emission line.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
49
Electromagnetic radiation
A) has only the properties of waves.
B) can behave both as a wave and as a particle.
C) can only travel in a dense medium.
D) is the same as a sound wave.
E) has nothing in common with radio waves.
A) has only the properties of waves.
B) can behave both as a wave and as a particle.
C) can only travel in a dense medium.
D) is the same as a sound wave.
E) has nothing in common with radio waves.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
50
What is true of a blackbody?
A) Its energy is not a continuum.
B) It has a complete absence of thermal energy.
C) Its energy peaks at the wavelength determined by its temperature.
D) If its temperature doubled, the peak in its radiation curve would be doubled in wavelength.
E) It appears black to us, regardless of its temperature.
A) Its energy is not a continuum.
B) It has a complete absence of thermal energy.
C) Its energy peaks at the wavelength determined by its temperature.
D) If its temperature doubled, the peak in its radiation curve would be doubled in wavelength.
E) It appears black to us, regardless of its temperature.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
51
A frequency of one hundred means the wave is vibrating one hundred million times per second; this is a typical carrier frequency for FM (frequency modulation) radio.
A) millihertz
B) gigahertz
C) hertz
D) megahertz
E) kilohertz
A) millihertz
B) gigahertz
C) hertz
D) megahertz
E) kilohertz

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
52
Increasing the temperature of a blackbody by a factor of 3 will increase its energy by a factor of
A) 3.
B) 6.
C) 9.
D) 12.
E) 81.
A) 3.
B) 6.
C) 9.
D) 12.
E) 81.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
53
According to the Zeeman effect, the splitting of a sunspot's spectral lines is due to
A) temperature variations.
B) a Doppler shift.
C) their radial velocity.
D) their rapid rotation.
E) their magnetic fields.
A) temperature variations.
B) a Doppler shift.
C) their radial velocity.
D) their rapid rotation.
E) their magnetic fields.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
54
The observed spectral lines of a star are all shifted towards the red end of the spectrum. Which statement is true?
A) The star has a radial velocity towards us.
B) The second law of Kirchhoff explains this.
C) The star is not rotating.
D) This is an example of the photoelectric effect.
E) This is an example of the Doppler effect.
A) The star has a radial velocity towards us.
B) The second law of Kirchhoff explains this.
C) The star is not rotating.
D) This is an example of the photoelectric effect.
E) This is an example of the Doppler effect.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
55
If a star was the same size as our Sun, but was 81 times more luminous, it must be
A) twice as hot as our Sun.
B) three times hotter than the Sun.
C) four times hotter than the Sun.
D) nine times hotter than the Sun.
E) 81 times hotter than the Sun.
A) twice as hot as our Sun.
B) three times hotter than the Sun.
C) four times hotter than the Sun.
D) nine times hotter than the Sun.
E) 81 times hotter than the Sun.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
56
If a source of light is approaching us at 3,000 km/sec, then all its waves are
A) blueshifted by 1%.
B) blueshifted out of the visible spectrum into the ultraviolet.
C) redshifted by 1%.
D) redshifted out of the visible into the infrared.
E) not affected, as c is constant regardless of the direction of motion.
A) blueshifted by 1%.
B) blueshifted out of the visible spectrum into the ultraviolet.
C) redshifted by 1%.
D) redshifted out of the visible into the infrared.
E) not affected, as c is constant regardless of the direction of motion.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
57
In the Kelvin scale, absolute zero lies at
A) - 373 degrees C.
B) 273 degrees C.
C) zero K.
D) Both A and B are correct.
E) Both A and C are correct.
A) - 373 degrees C.
B) 273 degrees C.
C) zero K.
D) Both A and B are correct.
E) Both A and C are correct.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
58
In general, the spectral lines of molecules are
A) less complex than those of atoms.
B) only absorption lines.
C) more complex than those of atoms.
D) the same as the atoms they contain.
E) nonexistent.
A) less complex than those of atoms.
B) only absorption lines.
C) more complex than those of atoms.
D) the same as the atoms they contain.
E) nonexistent.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
59
The total energy radiated by a blackbody depends on
A) the fourth root of its temperature.
B) the cube of its temperature.
C) the square of its temperature.
D) the fourth power of its temperature.
E) the square root of its temperature.
A) the fourth root of its temperature.
B) the cube of its temperature.
C) the square of its temperature.
D) the fourth power of its temperature.
E) the square root of its temperature.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
60
If the rest wavelength of a certain line is 600 nm, but we observe it at 594 nm, then
A) the source is getting 1% hotter as we watch.
B) the source is approaching us at 0.1% of the speed of light.
C) the source is receding from us at 10% of the speed of light.
D) the source is approaching us at 1% of the speed of light.
E) the source is spinning very rapidly, at 1% of the speed of light.
A) the source is getting 1% hotter as we watch.
B) the source is approaching us at 0.1% of the speed of light.
C) the source is receding from us at 10% of the speed of light.
D) the source is approaching us at 1% of the speed of light.
E) the source is spinning very rapidly, at 1% of the speed of light.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
61
The product of the wavelength times the frequency of a wave is its .

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
62
The common element discovered in the Sun's spectrum before it was found here is
.
.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
63
The Sun's blackbody curve peaks in the portion of the spectrum.
A) ultraviolet
B) X- ray
C) radio
D) infrared
E) visible
A) ultraviolet
B) X- ray
C) radio
D) infrared
E) visible

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
64
Stefan's law notes that total energy radiated is proportional to the _ power of the temperature of the blackbody.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
65
An electron has a electric charge.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
66
A wave with a period of .01 seconds has a frequency of Hz.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
67
Stars that appear blue or white in color are than our yellow Sun.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
68
The energy of the photon depends on its .

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
69
Fraunhofer was the German astronomer who first noted _ lines in the Sun's spectrum.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
70
According to Wein's law, the wavelength of the peak energy will be if the temperature of the blackbody is doubled.
A) doubled
B) quadrupled
C) quartered
D) unchanged
E) halved
A) doubled
B) quadrupled
C) quartered
D) unchanged
E) halved

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
71
The common element with bright red, blue- green, and violet emission lines is
A) hydrogen.
B) nitrogen.
C) oxygen.
D) carbon.
E) helium.
A) hydrogen.
B) nitrogen.
C) oxygen.
D) carbon.
E) helium.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
72
The most energetic photons are
A) infrared.
B) radio.
C) X- rays.
D) gamma rays.
E) visible.
A) infrared.
B) radio.
C) X- rays.
D) gamma rays.
E) visible.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
73
In electromagnetic waves, the electric and magnetic fields vibrate to each other.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
74
When an electron moves from a lower to a higher energy state, a photon is .

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
75
A dense, hot body will give off a(n) spectrum.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
76
A featureless spectrum, such as a rainbow, is said to be .

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
77
The distance from a wave's crest to its undisturbed position is the _ .

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
78
An FM station broadcasts at a frequency of 100 MHz. The wavelength of its carrier wave is
.
.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
79
A wave with a frequency of 2 Hz will have a period of .

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
80
Knowing the peak emission wavelength of a blackbody allows you to determine its
.
.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck


