Deck 41: Nuclear Physics and Radioactivity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/82
Play
Full screen (f)
Deck 41: Nuclear Physics and Radioactivity
1
The atomic mass unit is defined as
A)the mass of a proton.
B)the mass of a neutron.
C)the mass of an electron.
D)the mass of a hydrogen-1 atom.
E)one twelfth the mass of a carbon-12 atom.
A)the mass of a proton.
B)the mass of a neutron.
C)the mass of an electron.
D)the mass of a hydrogen-1 atom.
E)one twelfth the mass of a carbon-12 atom.
one twelfth the mass of a carbon-12 atom.
2
The nuclei of different isotopes of a given element have the same number of
A)protons.
B)neutrons.
C)nucleons.
D)electrons.
E)positrons.
A)protons.
B)neutrons.
C)nucleons.
D)electrons.
E)positrons.
protons.
3
Heavier stable nuclei tend to have
A)half as many protons as neutrons.
B)the same number of neutrons and protons.
C)more neutrons than protons.
D)no trend in relative number of neutrons and protons.
E)more protons than neutrons.
A)half as many protons as neutrons.
B)the same number of neutrons and protons.
C)more neutrons than protons.
D)no trend in relative number of neutrons and protons.
E)more protons than neutrons.
more neutrons than protons.
4
The proton is slightly more massive than the neutron.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
5
Except for the lightest nuclei, the average binding energy per nucleon is about
A)12 MeV.
B)4 MeV.
C)6 MeV.
D)10 MeV.
E)8 MeV.
A)12 MeV.
B)4 MeV.
C)6 MeV.
D)10 MeV.
E)8 MeV.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
6
Going from medium mass nuclei to a heavy nuclei, the average binding energy per nucleon
A)decreases.
B)behaves randomly.
C)remains constant.
D)increases.
E)doubles.
A)decreases.
B)behaves randomly.
C)remains constant.
D)increases.
E)doubles.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
7
If an atom's atomic number is given by Z, its atomic mass by A, and its neutron number by N, which of the following is correct?
A)N = A + Z
B)N = Z - A
C)N = A - Z
D)N = 2A + Z
E)N = A + 2Z
A)N = A + Z
B)N = Z - A
C)N = A - Z
D)N = 2A + Z
E)N = A + 2Z

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
8
All nuclei of a given element have the same number of neutrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
9
The mass of an atom is
A)approximately equally divided between protons and electrons.
B)approximately equally divided between neutrons, protons, and electrons.
C)evenly divided between the nucleus and the surrounding electron cloud.
D)concentrated in the cloud of electrons surrounding the nucleus.
E)concentrated in the nucleus.
A)approximately equally divided between protons and electrons.
B)approximately equally divided between neutrons, protons, and electrons.
C)evenly divided between the nucleus and the surrounding electron cloud.
D)concentrated in the cloud of electrons surrounding the nucleus.
E)concentrated in the nucleus.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
10
The symbols Z, A, and N respectively stand for
A)neutron number, atomic mass number, atomic number.
B)atomic number, atomic mass number, neutron number.
C)atomic number, neutron number, atomic mass number.
D)atomic mass number, atomic number, neutron number.
E)atomic mass number, proton number, nucleon number.
A)neutron number, atomic mass number, atomic number.
B)atomic number, atomic mass number, neutron number.
C)atomic number, neutron number, atomic mass number.
D)atomic mass number, atomic number, neutron number.
E)atomic mass number, proton number, nucleon number.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
11
Compared to the masses of its separate protons and neutrons, the total mass of a stable nucleus is always
A)less.
B)the same.
C)greater.
D)zero.
E)varies from atom to atom.
A)less.
B)the same.
C)greater.
D)zero.
E)varies from atom to atom.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
12
When nucleons join to form a stable nucleus, energy is
A)destroyed.
B)absorbed.
C)released.
D)not transferred.
E)either absorbed or released depending on nucleons involved.
A)destroyed.
B)absorbed.
C)released.
D)not transferred.
E)either absorbed or released depending on nucleons involved.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
13
The atomic number is equal to the number of what particles in the nucleus?
A)protons
B)neutrons
C)nucleons
D)electrons
E)positrons.
A)protons
B)neutrons
C)nucleons
D)electrons
E)positrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
14
The strong nuclear force acts only on neutral particles.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
15
The difference in the masses of a neutron and proton is
A)one.
B)equal to the mass of an electron.
C)less than the mass of an electron.
D)more than the mass of an electron.
E)zero.
A)one.
B)equal to the mass of an electron.
C)less than the mass of an electron.
D)more than the mass of an electron.
E)zero.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
16
Compared to the electrostatic force, the nuclear force between adjacent protons in a nucleus is
A)much weaker.
B)only slightly weaker.
C)about the same size.
D)only slightly larger.
E)much larger.
A)much weaker.
B)only slightly weaker.
C)about the same size.
D)only slightly larger.
E)much larger.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
17
A nucleon is
A)a neutron or a proton.
B)a nucleus made of neutrons only.
C)a neutron or proton when in the nucleus.
D)a neutron, proton, or positron when in the nucleus.
E)a neutron or positron when in the nucleus.
A)a neutron or a proton.
B)a nucleus made of neutrons only.
C)a neutron or proton when in the nucleus.
D)a neutron, proton, or positron when in the nucleus.
E)a neutron or positron when in the nucleus.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
18
Atoms with the same atomic number but with different numbers of neutrons are referred to as
A)positrons.
B)nucleons.
C)nuclides.
D)isotopes.
E)none of the given answers
A)positrons.
B)nucleons.
C)nuclides.
D)isotopes.
E)none of the given answers

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
19
The mass number is equal to the number of what particles in the nucleus?
A)protons
B)neutrons
C)nucleons
D)electrons
E)positrons.
A)protons
B)neutrons
C)nucleons
D)electrons
E)positrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
20
The competition between the electrostatic force between the protons in a nucleus and the strong nuclear force determines whether a nucleus is stable.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
21
When a β+ particle is emitted from an unstable nucleus, the atomic number of the nucleus
A)increases by 2.
B)increases by 1.
C)decreases by 1.
D)decreases by 2.
E)does not change.
A)increases by 2.
B)increases by 1.
C)decreases by 1.
D)decreases by 2.
E)does not change.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
22
There is a limit to the size of a stable nucleus because of
A)the limited range of the strong nuclear force.
B)the weakness of the electrostatic force.
C)the weakness of the gravitational force.
D)the weakness of the weak nuclear force.
E)none of the given answers
A)the limited range of the strong nuclear force.
B)the weakness of the electrostatic force.
C)the weakness of the gravitational force.
D)the weakness of the weak nuclear force.
E)none of the given answers

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
23
In beta decay, it is found that the energy of the emitted electrons varies and is always less than what is calculated on the basis of the parent and daughter nuclei. This is evidence for the existence of another particle called the
A)gluon.
B)meson.
C)neutrino.
D)quark.
E)positrons.
A)gluon.
B)meson.
C)neutrino.
D)quark.
E)positrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
24
In beta minus decay, the number of protons in the nucleus
A)is decreased by 1.
B)is decreased by 2.
C)is increased by 1.
D)is increased by 2.
E)remains unchanged.
A)is decreased by 1.
B)is decreased by 2.
C)is increased by 1.
D)is increased by 2.
E)remains unchanged.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
25
In all three types of radioactive decay, what value is conserved in addition to electric charge, energy, and momentum?
A)atomic number
B)neutron number
C)nucleon number
D)none of the given answers
A)atomic number
B)neutron number
C)nucleon number
D)none of the given answers

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
26
The nuclei of 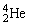 are also known as
are also known as
A)α particles.
B)β particles.
C)γ rays.
D)X-rays.
E)K-rays.
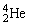 are also known as
are also known asA)α particles.
B)β particles.
C)γ rays.
D)X-rays.
E)K-rays.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
27
A β- decay occurs in an unstable nucleus when
A)a proton is converted to an electron by the strong force.
B)a proton is converted to a neutron by the strong force.
C)a neutron is converted to a proton by the weak force.
D)a neutron is converted to an alpha particle by the weak force.
E)a neutron is converted to a beta particle by the weak force.
A)a proton is converted to an electron by the strong force.
B)a proton is converted to a neutron by the strong force.
C)a neutron is converted to a proton by the weak force.
D)a neutron is converted to an alpha particle by the weak force.
E)a neutron is converted to a beta particle by the weak force.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
28
When a gamma ray is emitted from an unstable nucleus,
A)the number of neutrons and the number of protons drop by two.
B)the number of neutrons drops by one and the number of protons increases by one.
C)the number of protons drops by one and the number of neutrons increases by one.
D)there is no change in either the number of neutrons or the number of protons.
E)none of the given answers
A)the number of neutrons and the number of protons drop by two.
B)the number of neutrons drops by one and the number of protons increases by one.
C)the number of protons drops by one and the number of neutrons increases by one.
D)there is no change in either the number of neutrons or the number of protons.
E)none of the given answers

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
29
Gamma rays can penetrate
A)air only.
B)a piece of paper.
C)several millimeters of aluminum.
D)several centimeters of lead.
E)several meters of iron.
A)air only.
B)a piece of paper.
C)several millimeters of aluminum.
D)several centimeters of lead.
E)several meters of iron.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
30
In beta minus decay, the number of neutrons in the nucleus
A)is decreased by 1.
B)is decreased by 2.
C)is increased by 1.
D)is increased by 2.
E)remains unchanged.
A)is decreased by 1.
B)is decreased by 2.
C)is increased by 1.
D)is increased by 2.
E)remains unchanged.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
31
In alpha decay, the Q-value and the kinetic energy of the alpha particle
A)have no trend in the relative size.
B)are different with the Q-value larger.
C)are different with the kinetic energy larger.
D)are the same.
E)cannot be determined.
A)have no trend in the relative size.
B)are different with the Q-value larger.
C)are different with the kinetic energy larger.
D)are the same.
E)cannot be determined.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements concerning the nuclear force is false?
A)The nuclear force is very short-ranged.
B)The nuclear force is very weak and much smaller in relative magnitude than the electrostatic and gravitational forces.
C)The nuclear force is attractive and not repulsive.
D)The nuclear force acts on both protons and neutrons.
E)The nuclear is one of only four known types of forces in nature.
A)The nuclear force is very short-ranged.
B)The nuclear force is very weak and much smaller in relative magnitude than the electrostatic and gravitational forces.
C)The nuclear force is attractive and not repulsive.
D)The nuclear force acts on both protons and neutrons.
E)The nuclear is one of only four known types of forces in nature.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
33
When an unstable nucleus decays by emitting an alpha particle, the atomic number of the nucleus
A)increases by 4.
B)increases by 2.
C)decreases by 2.
D)decreases by 4.
E)remains unchanged.
A)increases by 4.
B)increases by 2.
C)decreases by 2.
D)decreases by 4.
E)remains unchanged.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
34
When an unstable nucleus decays by emitting an alpha particle, the atomic mass number of the nucleus
A)increases by 4.
B)increases by 2.
C)decreases by 2.
D)decreases by 4.
E)remains unchanged.
A)increases by 4.
B)increases by 2.
C)decreases by 2.
D)decreases by 4.
E)remains unchanged.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
35
A β- particle is also known as
A)an electron.
B)a protron.
C)a positron.
D)a helium nucleus.
E)a photon.
A)an electron.
B)a protron.
C)a positron.
D)a helium nucleus.
E)a photon.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
36
Beta rays can penetrate
A)air only.
B)a piece of paper.
C)several millimeters of aluminum.
D)several centimeters of lead.
E)several meters of iron.
A)air only.
B)a piece of paper.
C)several millimeters of aluminum.
D)several centimeters of lead.
E)several meters of iron.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is most nearly the same as a gamma ray?
A)an alpha particle
B)a beta ray
C)visible light
D)a proton
E)a neutron
A)an alpha particle
B)a beta ray
C)visible light
D)a proton
E)a neutron

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
38
A β+ particle is also known as
A)an electron.
B)a proton.
C)a positron.
D)a helium nucleus.
E)a photon.
A)an electron.
B)a proton.
C)a positron.
D)a helium nucleus.
E)a photon.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
39
Alpha rays can penetrate
A)air only.
B)a piece of paper.
C)several millimeters of aluminum.
D)several centimeters of lead.
E)several meters of iron.
A)air only.
B)a piece of paper.
C)several millimeters of aluminum.
D)several centimeters of lead.
E)several meters of iron.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
40
Gamma rays are emitted
A)often after beta decay.
B)often after alpha decay.
C)often from metastable states.
D)in all of the above.
E)in none of the above.
A)often after beta decay.
B)often after alpha decay.
C)often from metastable states.
D)in all of the above.
E)in none of the above.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
41
The symbol for a certain isotope of polonium is 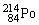 . How many neutrons are there in the nucleus of this isotope?
. How many neutrons are there in the nucleus of this isotope?
A)84
B)130
C)214
D)298
E)314
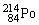 . How many neutrons are there in the nucleus of this isotope?
. How many neutrons are there in the nucleus of this isotope?A)84
B)130
C)214
D)298
E)314

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
42
The atomic number and mass number for calcium 39 are 20 and 39, respectively.
(a) How many nucleons are in one atom?
(b) How many electrons are in one atom?
(c) How many protons are in one atom?
(d) How many neutrons are in one atom?
(a) How many nucleons are in one atom?
(b) How many electrons are in one atom?
(c) How many protons are in one atom?
(d) How many neutrons are in one atom?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
43
In a 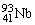 nucleus, the number of protons, neutrons, and electrons is
nucleus, the number of protons, neutrons, and electrons is
A)41, 52, 93.
B)41, 52, 52.
C)41, 52, 41.
D)41, 52, 0.
E)52, 41, 0.
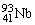 nucleus, the number of protons, neutrons, and electrons is
nucleus, the number of protons, neutrons, and electrons isA)41, 52, 93.
B)41, 52, 52.
C)41, 52, 41.
D)41, 52, 0.
E)52, 41, 0.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
44
The mass of 1H is 1.007825 u and the mass of 1n is 1.008665 u. The mass of 12C is 12.000000 u, of 13N is 13.005739 u, and of 14N is 14.003074 u. What is the binding energy of the last proton in 13N?
A)2.5 MeV
B)1.9 MeV
C)9.8 MeV
D)10.6 MeV
E)3.8 MeV
A)2.5 MeV
B)1.9 MeV
C)9.8 MeV
D)10.6 MeV
E)3.8 MeV

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
45
An element has an atomic mass number of 14 and atomic number 6.
(a) How many protons does it have?
(b) How many neutrons does it have?
(a) How many protons does it have?
(b) How many neutrons does it have?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
46
What is the nuclear radius of 90Sr?
A)4.0 × 10-15 m
B)1.2 × 10-15 m
C)1.1 × 10-13 m
D)5.4 × 10-15 m
E)3.3 × 10-13 m
A)4.0 × 10-15 m
B)1.2 × 10-15 m
C)1.1 × 10-13 m
D)5.4 × 10-15 m
E)3.3 × 10-13 m

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
47
The type of detector that uses liquid hydrogen is a
A)Geiger tube.
B)scintillation counter.
C)cloud chamber.
D)bubble chamber.
E)spark chamber.
A)Geiger tube.
B)scintillation counter.
C)cloud chamber.
D)bubble chamber.
E)spark chamber.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
48
The mass of 1H is 1.007825 u and the mass of 1n is 1.008665 u. The mass of 12C is 12.000000 u, of 13N is 13.005739 u, and of 14N is 14.003074 u. What is the binding energy of the last neutron in 14N?
A)10.6 MeV
B)9.8 MeV
C)1.9 MeV
D)2.5 MeV
E)3.8 MeV
A)10.6 MeV
B)9.8 MeV
C)1.9 MeV
D)2.5 MeV
E)3.8 MeV

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
49
An aluminum foil 0.10 mm thick has how many aluminum atoms per square cm? (The density of aluminum is 2.7 × 103 kg/ m3 and the atomic mass of aluminum is 27.)
A)6 × 1017
B)6 × 1020
C)6 × 1024
D)6 × 109
E)6 × 1030
A)6 × 1017
B)6 × 1020
C)6 × 1024
D)6 × 109
E)6 × 1030

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
50
The neutral deuterium atom, 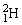 , has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the
, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the 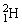 nucleus? 1 u = 931.494 MeV/c2.
nucleus? 1 u = 931.494 MeV/c2.
A)1.1 MeV
B)1.7 MeV
C)2.2 MeV
D)2.9 MeV
E)3.4 MeV
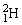 , has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the
, has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. What is the binding energy of the 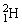 nucleus? 1 u = 931.494 MeV/c2.
nucleus? 1 u = 931.494 MeV/c2.A)1.1 MeV
B)1.7 MeV
C)2.2 MeV
D)2.9 MeV
E)3.4 MeV

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
51
The symbol for a certain isotope of radium is 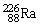 . How many nucleons are there in the nucleus of this isotope?
. How many nucleons are there in the nucleus of this isotope?
A)88
B)138
C)214
D)226
E)314
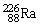 . How many nucleons are there in the nucleus of this isotope?
. How many nucleons are there in the nucleus of this isotope?A)88
B)138
C)214
D)226
E)314

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
52
In radioactive dating, carbon-14 is often used. This nucleus emits a single beta particle when it decays. When this happens, the resulting nucleus is
A)still carbon-14.
B)boron-14.
C)nitrogen-14.
D)carbon-15.
E)carbon-13.
A)still carbon-14.
B)boron-14.
C)nitrogen-14.
D)carbon-15.
E)carbon-13.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
53
What happens to the half-life of a radioactive substance as it decays?
A)It remains constant.
B)It increases.
C)It decreases.
D)It could do any of these.
A)It remains constant.
B)It increases.
C)It decreases.
D)It could do any of these.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
54
Cloud chambers have been replaced by bubble chambers because
A)the radioactive clouds were too dangerous to work with.
B)the density of fluids is greater than the density of vapors.
C)bubble chambers tend to be larger and more expensive.
D)gamma rays are visible in bubble chambers, but not in cloud chambers.
A)the radioactive clouds were too dangerous to work with.
B)the density of fluids is greater than the density of vapors.
C)bubble chambers tend to be larger and more expensive.
D)gamma rays are visible in bubble chambers, but not in cloud chambers.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
55
The symbol for a certain isotope of strontium is 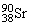 . What is the mass number of this isotope?
. What is the mass number of this isotope?
A)38
B)52
C)88
D)90
E)128
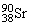 . What is the mass number of this isotope?
. What is the mass number of this isotope?A)38
B)52
C)88
D)90
E)128

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
56
If a nucleus had a diameter of 8.0 × 10-15 m, what would its mass be in atomic mass units?
A)7
B)296
C)37
D)64
E)128
A)7
B)296
C)37
D)64
E)128

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
57
The type of detector that uses a magnetic field to curve charged particles is a
A)Geiger tube.
B)scintillation counter.
C)cloud chamber.
D)bubble chamber.
E)spark chamber.
A)Geiger tube.
B)scintillation counter.
C)cloud chamber.
D)bubble chamber.
E)spark chamber.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
58
What is the binding energy per nucleon for 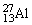 ? The neutral
? The neutral 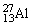 atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. 1 u = 931.494 MeV/c2.
atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. 1 u = 931.494 MeV/c2.
A)8.3 MeV
B)6.7 MeV
C)5.4 MeV
D)3.4 MeV
E)2.8 MeV
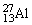 ? The neutral
? The neutral 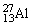 atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. 1 u = 931.494 MeV/c2.
atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u. 1 u = 931.494 MeV/c2.A)8.3 MeV
B)6.7 MeV
C)5.4 MeV
D)3.4 MeV
E)2.8 MeV

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
59
The mass of 1H is 1.007825 u and the mass of 1n is 1.008665 u. The mass of 12C is 12.000000 u. What is the total binding energy of 12C?
A)7.7 MeV
B)92 MeV
C)0.099 MeV
D)46 MeV
E)15 MeV
A)7.7 MeV
B)92 MeV
C)0.099 MeV
D)46 MeV
E)15 MeV

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
60
What is the density of the nucleus of 90Sr?
A)greater than 3 × 1020 kg/ m3 m3
B)90 kg/ m3
C)2.1 × 1019 kg/ m3
D)2.0 × 1015 kg/ m3
E)2.3 × 1017 kg/ m3
A)greater than 3 × 1020 kg/ m3 m3
B)90 kg/ m3
C)2.1 × 1019 kg/ m3
D)2.0 × 1015 kg/ m3
E)2.3 × 1017 kg/ m3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
61
An atom has 98 protons and 249 nucleons. If it undergoes alpha decay, what are the number of protons and nucleons, respectively, in the daughter nucleus?
A)100, 245
B)94, 247
C)96, 245
D)96, 247
E)100, 249
A)100, 245
B)94, 247
C)96, 245
D)96, 247
E)100, 249

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
62
A stationary plutonium-239 nucleus decays into a uranium-235 nucleus plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values: 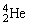 , 4.002603 u;
, 4.002603 u; 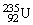 , 235.043924 u, what is the kinetic energy of the
, 235.043924 u, what is the kinetic energy of the 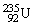 nucleus? 1 u =931.494 MeV/c2.
nucleus? 1 u =931.494 MeV/c2.
A)0.0829 MeV
B)0.0837 MeV
C)0.0852 MeV
D)0.0863 MeV
E)0.0877 MeV
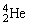 , 4.002603 u;
, 4.002603 u; 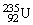 , 235.043924 u, what is the kinetic energy of the
, 235.043924 u, what is the kinetic energy of the 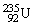 nucleus? 1 u =931.494 MeV/c2.
nucleus? 1 u =931.494 MeV/c2.A)0.0829 MeV
B)0.0837 MeV
C)0.0852 MeV
D)0.0863 MeV
E)0.0877 MeV

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
63
Francium-223 decays to radium-223 by emitting what kind of nuclear radiation?
A)Alpha
B)Beta minus
C)Beta plus
D)Gamma
E)X-rays.
A)Alpha
B)Beta minus
C)Beta plus
D)Gamma
E)X-rays.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
64
Americium-243 has a decay constant of 9.39 × 10-5years-1. How long will it take for a sample of americium to lose one-third of its nuclei?
A)2960 s
B)10.6 × 103 years
C)4.32 × 103 years
D)6.44 × 103 years
E)7.84 × 103 years
A)2960 s
B)10.6 × 103 years
C)4.32 × 103 years
D)6.44 × 103 years
E)7.84 × 103 years

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
65
Radium-226 decays into radon-222 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2. The relevant mass values are: 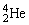 , 4.002603 u;
, 4.002603 u; 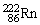 , 222.017570 u;
, 222.017570 u; 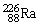 , 226.025402 u.
, 226.025402 u.
A)4.24 MeV
B)3.76 MeV
C)4.87 MeV
D)5.05 MeV
E)5.39 MeV
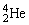 , 4.002603 u;
, 4.002603 u; 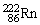 , 222.017570 u;
, 222.017570 u; 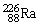 , 226.025402 u.
, 226.025402 u.A)4.24 MeV
B)3.76 MeV
C)4.87 MeV
D)5.05 MeV
E)5.39 MeV

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
66
A radioactive sample has a half-life of 10 min. What fraction of the sample is left after 40 min?
A)1/2
B)1/4
C)1/8
D)1/16
E)1/32
A)1/2
B)1/4
C)1/8
D)1/16
E)1/32

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
67
241Am decays by alpha particle emission. The daughter isotope in this case is (the atomic numbers of Np, Pu, Am, Cm, and Bk are 91, 92, 93, 94, and 95 respectively)
A)(239Np.)
B)(237Np.)
C)(245Bk.)
D)(235Bk.)
E)not given.
A)(239Np.)
B)(237Np.)
C)(245Bk.)
D)(235Bk.)
E)not given.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
68
Rutherfordium-261 has a half-life of 1.08 min. What is the decay constant of rutherfordium?
A)0.0107 s-1
B)0.0154 s-1
C)1.87 s-1
D)2.91 s-1
E)0.344 s-1
A)0.0107 s-1
B)0.0154 s-1
C)1.87 s-1
D)2.91 s-1
E)0.344 s-1

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
69
Plutonium-239 decays into uranium-235 plus an alpha particle. The energy released in the process is 5.24 MeV. Given the following mass values: 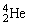 , 4.002603 u;
, 4.002603 u; 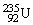 , 235.043924 u; what is the mass value of
, 235.043924 u; what is the mass value of 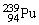 ? 1 u = 931.494 MeV/c2.
? 1 u = 931.494 MeV/c2.
A)239.052157 u
B)239.027750 u
C)239.001889 u
D)238.9991889 u
E)238.988842 u
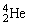 , 4.002603 u;
, 4.002603 u; 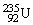 , 235.043924 u; what is the mass value of
, 235.043924 u; what is the mass value of 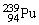 ? 1 u = 931.494 MeV/c2.
? 1 u = 931.494 MeV/c2.A)239.052157 u
B)239.027750 u
C)239.001889 u
D)238.9991889 u
E)238.988842 u

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
70
A carbon-14 nucleus decays to a nitrogen-14 nucleus by beta decay. How much energy (in MeV) is released if carbon-14 has a mass of 14.003242 u and nitrogen-14 has a mass of 14.003074 u?
A)0.00157 MeV
B)0.0157 MeV
C)0.157 MeV
D)1.57 MeV
E)15.7 MeV
A)0.00157 MeV
B)0.0157 MeV
C)0.157 MeV
D)1.57 MeV
E)15.7 MeV

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
71
Uranium-238 decays into thorium-234 plus an alpha particle. How much energy is released in this process? 1 u = 931.494 MeV/c2. The relevant mass values are: 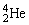 , 4.002603 u;
, 4.002603 u; 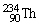 , 234.043583 u;
, 234.043583 u; 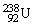 , 238.050786 u.
, 238.050786 u.
A)4.28 MeV
B)3.76 MeV
C)3.18 MeV
D)2.89 MeV
E)5.05 MeV
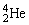 , 4.002603 u;
, 4.002603 u; 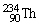 , 234.043583 u;
, 234.043583 u; 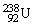 , 238.050786 u.
, 238.050786 u.A)4.28 MeV
B)3.76 MeV
C)3.18 MeV
D)2.89 MeV
E)5.05 MeV

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
72
An element with atomic number 6 undergoes β- decay. Its atomic number is now
A)7.
B)6.
C)5.
D)3.
E)2.
A)7.
B)6.
C)5.
D)3.
E)2.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
73
A certain substance has a half-life of 5.0 hours. How many nuclei of the substance are required to give an initial activity of 6.0 μCi? 1 Ci = 3.7 × 1010 Bq.
A)5.8 × 109 nuclei
B)8.5 × 108 nuclei
C)6.3 × 108 nuclei
D)3.2 × 109 nuclei
E)2.4 × 109 nuclei
A)5.8 × 109 nuclei
B)8.5 × 108 nuclei
C)6.3 × 108 nuclei
D)3.2 × 109 nuclei
E)2.4 × 109 nuclei

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
74
The decay constant of radon is 0.181 day s-1. A sample of radon contains 6.00 × 108 radon atoms. How many atoms are left after 10.0 days?
A)9.82 × 107
B)7.67 × 107
C)8.34 × 108
D)7.29 × 108
E)8.56 × 108
A)9.82 × 107
B)7.67 × 107
C)8.34 × 108
D)7.29 × 108
E)8.56 × 108

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
75
Polonium-216 decays to lead-212 by emitting what kind of nuclear radiation?
A)Alpha
B)Beta minus
C)Beta plus
D)Gamma
E)X-rays.
A)Alpha
B)Beta minus
C)Beta plus
D)Gamma
E)X-rays.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
76
An element with atomic number 88 goes through alpha decay. Its atomic number is now
A)80.
B)82.
C)84.
D)86.
E)88.
A)80.
B)82.
C)84.
D)86.
E)88.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
77
Rutherfordium-261 has a half-life of 1.08 min. How long will it take for a sample of rutherfordium to lose one-third of its nuclei?
A)1.02 min
B)1.62 min
C)0.63 min
D)2.70 min
E)3.24 min
A)1.02 min
B)1.62 min
C)0.63 min
D)2.70 min
E)3.24 min

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
78
Fermium-253 has a half-life of 3.00 days. A sample of fermium has 7.37 × 107 nuclei. How long will it take for there to be only 3.36 × 106 fermium nuclei in this sample?
A)2.75 days
B)9.80 days
C)13.4 days
D)15.7 days
E)58.6 days
A)2.75 days
B)9.80 days
C)13.4 days
D)15.7 days
E)58.6 days

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
79
The number of radioactive nuclei in a particular sample decreases to one-eighth of its original number in 9 days. What is the half-life of these nuclei?
A)9/8 days
B)2 days
C)3 days
D)8 days
E)10 days
A)9/8 days
B)2 days
C)3 days
D)8 days
E)10 days

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
80
The radioactivity due to carbon-14 measured in a piece of a wooden casket from an ancient burial site was found to produce 20 counts per minute from a given sample, whereas the same amount of carbon from a piece of living wood produced 160 counts per minute. The half-life of carbon-14, a beta emitter, is 5730 years. Thus we would estimate the age of the artifact to be about
A)5,700 years.
B)11,500 years.
C)14,800 years.
D)17,200 years.
E)23,000 years.
A)5,700 years.
B)11,500 years.
C)14,800 years.
D)17,200 years.
E)23,000 years.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck


