Deck 20: Second Law of Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 20: Second Law of Thermodynamics
1
The entropy of an isolated system never changes.
False
2
The entropy of an isolated system never increases..
False
3
Sketch the schematic diagram of energy transfers for a heat engine.
See Fig. 20-2 in the text.
4
Describe the operation of an automobile internal combustion engine in terms of the Otto cycle.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
Give a general statement of the Second Law of Thermodynamics.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
Which has the larger entropy: a gas that occupies only half of a container, with the other half a vacuum, or the same gas occupying the entire container?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
The total entropy of any system plus that of its environment increases as a result of any natural process.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
The entropy of an isolated system never decreases.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
Entropy is a state function.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
Give the Kelvin-Planck statement of the Second Law of Thermodynamics.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
Give the Clausius statement of the Second Law of Thermodynamics.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
State the Third Law of Thermodynamics

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
Explain the following statement: "In any natural process, some energy becomes unavailable to do useful work."

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
Heat will flow spontaneously from a cold object to a hot object.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
Describe the Carnot cycle.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
A heat engine is any device that changes mechanical work into thermal energy.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
All reversible engines operating between the same two temperatures have the same efficiency.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
On a hot day, you open the refrigerator and experience a refreshing feeling when the cool air comes in contact with your body. You think to yourself "I think that I'll just keep the refrigerator door open and stand in front of the refrigerator all day today to stay cool. I might even bring a chair." Will this work? Explain why or why not.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
Sketch the schematic diagram of energy transfers for a refrigerator or air conditioner

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
No device is possible whose sole effect is to transform a given amount of heat completely into work.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
The coefficient of performance (COP) of a heat pump is defined as the ratio of
A)the heat delivered to the inside to the heat taken from the outside.
B)the heat taken from the outside to the heat delivered to the inside.
C)the heat delivered to the inside to the work done to move the heat.
D)the heat taken from the outside to the work done to move the heat.
E)the difference between the heat taken from the outside from the heat delivered to the inside to the work done to move the heat.
A)the heat delivered to the inside to the heat taken from the outside.
B)the heat taken from the outside to the heat delivered to the inside.
C)the heat delivered to the inside to the work done to move the heat.
D)the heat taken from the outside to the work done to move the heat.
E)the difference between the heat taken from the outside from the heat delivered to the inside to the work done to move the heat.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
One of the most efficient engines built so far has the following characteristics: combustion chamber temperature = 1900°C, exhaust temperature = 430°C, 7.0 × 109 cal of fuel produces 1.4 × 1010 J of work in one hour.
(a) What is the actual efficiency of this engine?
(b) What is the Carnot efficiency of the engine?
(c) What is the power output, in hp, of this engine?
(a) What is the actual efficiency of this engine?
(b) What is the Carnot efficiency of the engine?
(c) What is the power output, in hp, of this engine?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is an example of a reversible process?
A)An ice cube at 0°C melts while in a water bath at 0°C.
B)A hand pump is rapidly compressed and the pressure and temperature in the pump rise. The pump is held in position as the temperature drops to the ambient temperature.
C)A stick of dynamite explodes.
D)A high pressure air tank at room temperature has its valve opened and the gas in the tank rushes out until the pressure in the tank is equal to atmospheric pressure.
E)A car engine burns fuel to produce motion of the car and exhausts the 140°C waste products of the combustion into the 20°C atmosphere.
A)An ice cube at 0°C melts while in a water bath at 0°C.
B)A hand pump is rapidly compressed and the pressure and temperature in the pump rise. The pump is held in position as the temperature drops to the ambient temperature.
C)A stick of dynamite explodes.
D)A high pressure air tank at room temperature has its valve opened and the gas in the tank rushes out until the pressure in the tank is equal to atmospheric pressure.
E)A car engine burns fuel to produce motion of the car and exhausts the 140°C waste products of the combustion into the 20°C atmosphere.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
FIGURE 20-2 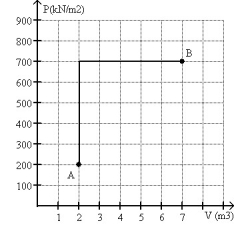
A monatomic ideal gas reversibly follows the process shown in Fig. 20-2 starting from state A at a temperature 300 K and ending at state B.
(a) What is the final temperature of the gas?
(b) What is the change in internal energy of the gas in going from state A to state B?
(c) What is the change in entropy of the gas in going from state A to state B?

A monatomic ideal gas reversibly follows the process shown in Fig. 20-2 starting from state A at a temperature 300 K and ending at state B.
(a) What is the final temperature of the gas?
(b) What is the change in internal energy of the gas in going from state A to state B?
(c) What is the change in entropy of the gas in going from state A to state B?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
From the energy that is input into an engine on each cycle, 500 kJ of mechanical work are produced and 600 kJ of heat are exhausted into the environment. What is the efficiency of the engine?
A)45.5%
B)28.6%
C)90.0%
D)75.0%
E)83.3%
A)45.5%
B)28.6%
C)90.0%
D)75.0%
E)83.3%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is a statement of the third law of thermodynamics?
A)If two objects are in equilibrium with a third, then they are in thermal equilibrium with one another.
B)The entropy of the universe cannot decrease.
C)The entropy of the universe cannot increase.
D)All reversible engines operating between the same two temperatures have the same efficiency.
E)It is impossible to lower the temperature of an object to absolute zero in a finite number of steps.
A)If two objects are in equilibrium with a third, then they are in thermal equilibrium with one another.
B)The entropy of the universe cannot decrease.
C)The entropy of the universe cannot increase.
D)All reversible engines operating between the same two temperatures have the same efficiency.
E)It is impossible to lower the temperature of an object to absolute zero in a finite number of steps.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
Natural processes tend to move toward a state of less disorder.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
A engine manufacturer makes the claim that the engine they have developed will, on each cycle, take 100 J of heat out of boiling water at 100°C, do mechanical work of 80 J, and exhaust 20 J of heat at 10°C. What, if anything, is wrong with this claim?
A)The heat exhausted must always be greater than the work done according to the second law of thermodynamics.
B)This engine violates the first law of thermodynamics because 100 J + 20 J ≠ 80 J
C)An engine would operate by taking in heat at the lower temperature and exhausting heat at the higher temperature.
D)The efficiency of this engine is greater than the ideal Carnot cycle efficiency.
E)There is nothing wrong with this claim because 100 J = 20 J + 80 J.
A)The heat exhausted must always be greater than the work done according to the second law of thermodynamics.
B)This engine violates the first law of thermodynamics because 100 J + 20 J ≠ 80 J
C)An engine would operate by taking in heat at the lower temperature and exhausting heat at the higher temperature.
D)The efficiency of this engine is greater than the ideal Carnot cycle efficiency.
E)There is nothing wrong with this claim because 100 J = 20 J + 80 J.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
A Carnot cycle consists of
A)two adiabats and two isobars.
B)two isobars and two isotherms.
C)two isotherms and two isomets.
D)two adiabats and two isotherms.
E)two adiabats and two isomets.
A)two adiabats and two isobars.
B)two isobars and two isotherms.
C)two isotherms and two isomets.
D)two adiabats and two isotherms.
E)two adiabats and two isomets.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
A heat engine has an efficiency of 35.0% and receives 150 J of heat per cycle.
(a) How much work does it perform in each cycle?
(b) How much heat does it exhaust in each cycle?
(a) How much work does it perform in each cycle?
(b) How much heat does it exhaust in each cycle?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
According to the second law of thermodynamics, for any process that may occur within an isolated system, which one of the choices applies?
A)Entropy remains constant.
B)Entropy increases.
C)Entropy decreases.
D)Both A and B are possible.
E)Both A and C are possible.
A)Entropy remains constant.
B)Entropy increases.
C)Entropy decreases.
D)Both A and B are possible.
E)Both A and C are possible.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
A coal-fired plant generates 600 MW of electric power. The plant uses 4.8 × 106 kg of coal each day. The heat of combustion of coal is 3.3 × 107 J/kg. The steam that drives the turbines is at a temperature of 300°C, and the exhaust water is at 37°C.
(a) What is the overall efficiency of the plant for generating electric power?
(b) What is the Carnot efficiency?
(c) How much thermal energy is exhausted each day?
(a) What is the overall efficiency of the plant for generating electric power?
(b) What is the Carnot efficiency?
(c) How much thermal energy is exhausted each day?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
If the theoretical efficiency of a Carnot engine is to be 100%, the heat sink must be
A)at absolute zero.
B)at 0°C.
C)at 100°C.
D)at 1000°C.
E)infinitely hot.
A)at absolute zero.
B)at 0°C.
C)at 100°C.
D)at 1000°C.
E)infinitely hot.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
If a system undergoes a reversible process,
A)it must be possible to restore the system to its original state.
B)it must be possible to restore the surroundings to their original state.
C)it must be possible to restore both the system and the surroundings to their original states.
D)it is impossible to restore either the the system or the surroundings to their original states.
E)the system must not interact with its surroundings.
A)it must be possible to restore the system to its original state.
B)it must be possible to restore the surroundings to their original state.
C)it must be possible to restore both the system and the surroundings to their original states.
D)it is impossible to restore either the the system or the surroundings to their original states.
E)the system must not interact with its surroundings.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
FIGURE 20-1 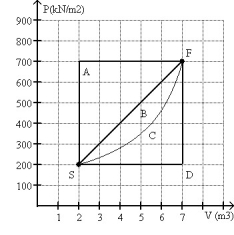
Which of the processes on an ideal gas shown in Fig. 20-1 results in the minimum change in entropy of the gas in changing the gas from state S to State F.
A)A
B)B
C)C
D)D
E)All processes result in the same change in entropy of the gas.

Which of the processes on an ideal gas shown in Fig. 20-1 results in the minimum change in entropy of the gas in changing the gas from state S to State F.
A)A
B)B
C)C
D)D
E)All processes result in the same change in entropy of the gas.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
The efficiency of a heat engine is defined as the ratio of
A)the heat input at the high temperature to the heat output at the low temperature.
B)the heat output at the low temperature to the heat input at the high temperature.
C)the work it does to the heat input at the high temperature.
D)the work it does to the heat output at the low temperature.
E)the work it does to the difference between the heat input from the heat output.
A)the heat input at the high temperature to the heat output at the low temperature.
B)the heat output at the low temperature to the heat input at the high temperature.
C)the work it does to the heat input at the high temperature.
D)the work it does to the heat output at the low temperature.
E)the work it does to the difference between the heat input from the heat output.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
In any natural process, some energy becomes unavailable to do useful work.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
The coefficient of performance (COP) of a refrigerator is defined as the ratio of
A)the heat removed from the inside to the heat expelled to the outside.
B)the heat expelled to the outside to the heat removed from the inside.
C)the heat removed from the inside to the work done to remove the heat.
D)the heat expelled to the outside to the work done to remove the heat.
E)the difference between the heat expelled to the outside from the heat removed from the inside to the work done to remove the heat.
A)the heat removed from the inside to the heat expelled to the outside.
B)the heat expelled to the outside to the heat removed from the inside.
C)the heat removed from the inside to the work done to remove the heat.
D)the heat expelled to the outside to the work done to remove the heat.
E)the difference between the heat expelled to the outside from the heat removed from the inside to the work done to remove the heat.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
The second law of thermodynamics leads us to conclude that
A)the total energy of the universe is constant.
B)disorder in the universe is increasing with the passage of time.
C)it is theoretically possible to convert heat into work with 100% efficiency.
D)the average temperature of the universe is increasing with the passage of time.
E)the average temperature of the universe is decreasing with the passage of time.
A)the total energy of the universe is constant.
B)disorder in the universe is increasing with the passage of time.
C)it is theoretically possible to convert heat into work with 100% efficiency.
D)the average temperature of the universe is increasing with the passage of time.
E)the average temperature of the universe is decreasing with the passage of time.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
Order in one part of the universe can only be produced at the expense of disorder in another part.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
A Carnot cycle operates with an efficiency 0.60 between a low temperature heat bath at 20.0°C and a high temperature heat bath. What is the temperature of the high temperature heat bath?
A)33.3°C
B)50.0°C
C)489°C
D)460°C
E)215°C
A)33.3°C
B)50.0°C
C)489°C
D)460°C
E)215°C

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
An ideal, reversible heat pump is taking heat from the outside air at -10.0°C and discharging it into the house at 18.0°C. What is the coefficient of performance of this heat pump?
A)10.4
B)9.44
C)0.644
D)0.533
E)0.0962
A)10.4
B)9.44
C)0.644
D)0.533
E)0.0962

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
A Carnot refrigerator has a coefficient of performance = 2.5. The refrigerator consumes 50 W of power. How much heat is removed from the interior of the refrigerator in 1 hour?
A)7.5 kJ
B)4.5 × 105 J
C)1.8 × 105 J
D)7.2 × 105 J
E)72 kJ
A)7.5 kJ
B)4.5 × 105 J
C)1.8 × 105 J
D)7.2 × 105 J
E)72 kJ

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
A heat engine operating at maximum efficiency has an efficiency of 25%. The temperature of the cold reservoir is 300 K. What is the temperature of the hot reservoir?
A)350 K
B)375 K
C)400 K
D)450 K
E)500 K
A)350 K
B)375 K
C)400 K
D)450 K
E)500 K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
The efficiency of a Carnot engine is 35.0%. What is the temperature of the cold reservoir if the temperature of the hot reservoir is 500 K?
A)95.0 K
B)175 K
C)325 K
D)269 K
E)231 K
A)95.0 K
B)175 K
C)325 K
D)269 K
E)231 K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
What is the Carnot cycle efficiency for a system that operates with low temperature reservoir at 20°C and a high temperature reservoir at 200°C?
A)0.620
B)0.380
C)0.288
D)0.411
E)0.900
A)0.620
B)0.380
C)0.288
D)0.411
E)0.900

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
A heat engine with an efficiency of 30.0% performs 2500 J of work. How much heat is discharged to the lower temperature reservoir?
A)5830 J
B)8330 J
C)750 J
D)1350 J
E)7080 J
A)5830 J
B)8330 J
C)750 J
D)1350 J
E)7080 J

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
A Carnot air conditioner operates between an indoor temperature of 20°C and an outdoor temperature of 39°C. How much energy does it need to remove 2000 J of heat from the interior of the house?
A)105 J
B)130 J
C)780 J
D)520 J
E)340 J
A)105 J
B)130 J
C)780 J
D)520 J
E)340 J

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
A heat pump has a coefficient of performance that is 60% of the Carnot heat pump coefficient of performance. The heat pump is used to heat a home to 24.0°C during the winter with the low temperature reservoir at the outdoor temperature. At which outdoor temperature would it be more efficient to add the energy directly to the interior of the home than use it to run the heat pump?
A)-25.2°C
B)-154°C
C)-40.0°C
D)-83.4°C
E)-4.00°C
A)-25.2°C
B)-154°C
C)-40.0°C
D)-83.4°C
E)-4.00°C

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
A perfect Carnot engine operates between the temperatures of 300K and 700K, drawing 60 kJ of heat from the 700K reservoir in each cycle. How much heat is dumped into the 300K reservoir in each cycle?
A)38 kJ
B)34 kJ
C)30 kJ
D)26 kJ
E)42 kJ
A)38 kJ
B)34 kJ
C)30 kJ
D)26 kJ
E)42 kJ

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
An air conditioner with a coefficient of performance of 3.5 uses 30 kW of power. How much power is it discharging to the outdoors?
A)30 kW
B)75 kW
C)105 kW
D)135 kW
E)210 kW
A)30 kW
B)75 kW
C)105 kW
D)135 kW
E)210 kW

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
A refrigerator has a coefficient of performance equal to 4.2. How much work must be done on the refrigerator in order to remove 250 J of heat from the interior?
A)60 J
B)120 J
C)250 J
D)480 J
E)1050 J
A)60 J
B)120 J
C)250 J
D)480 J
E)1050 J

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
What is the maximum theoretical efficiency possible for an engine operating between 100°C and 400°C?
A)25%
B)45%
C)55%
D)65%
E)75%
A)25%
B)45%
C)55%
D)65%
E)75%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
What is the coefficient of performance of a Carnot heat pump used to remove heat from ice at 0.0°C and add heat to a room at 20.0°C?
A)14.7
B)1.00
C)0.00
D)20.0
E)293
A)14.7
B)1.00
C)0.00
D)20.0
E)293

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
The compressor in a certain Carnot refrigerator performs 480 J of work to remove 150 J of heat from the interior of the refrigerator. How much heat must the coils behind the refrigerator discharge into the kitchen?
A)110 J
B)150 J
C)330 J
D)480 J
E)630 J
A)110 J
B)150 J
C)330 J
D)480 J
E)630 J

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
A perfect Carnot engine operates between 350 K and 600 K. The engine delivers 10 kJ of work in each cycle. How much heat is extracted from the 600 K reservoir in one cycle?
A)34 kJ
B)27 kJ
C)17 kJ
D)21 kJ
E)24 kJ
A)34 kJ
B)27 kJ
C)17 kJ
D)21 kJ
E)24 kJ

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
A Carnot cycle engine operates between a low temperature reservoir at 20°C and a high temperature reservoir at 800°C. If the engine is required to output 20.0 kJ of work per cycle, how much heat must the high temperature reservoir transfer to the engine during each cycle?
A)27.5 kJ
B)73.2 kJ
C)39.2 kJ
D)800 kJ
E)20.5 kJ
A)27.5 kJ
B)73.2 kJ
C)39.2 kJ
D)800 kJ
E)20.5 kJ

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
A heat engine operates between 800 K and 300 K, but its efficiency is only 80% of the maximum possible. What is the actual efficiency of the engine?
A)30%
B)70%
C)38%
D)50%
E)36%
A)30%
B)70%
C)38%
D)50%
E)36%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
A heat engine operating at maximum efficiency has an efficiency of 35.0%. The temperature of the hot reservoir is 700 K. What is the temperature of the cold reservoir?
A)200 K
B)245 K
C)350 K
D)455 K
E)600 K
A)200 K
B)245 K
C)350 K
D)455 K
E)600 K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
A certain engine extracts 1300 J of heat from a hot temperature reservoir and discharges 700 J of heat to a cold temperature reservoir. What is the efficiency of this engine?
A)46%
B)54%
C)86%
D)27%
E)13%
A)46%
B)54%
C)86%
D)27%
E)13%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
FIGURE 20-3 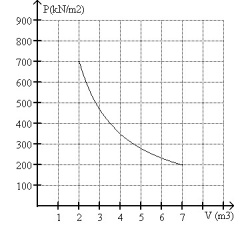
What is the change in entropy of 10.0 moles of monatomic ideal gas with a molar specific heat 3R/2 that reversibly undergoes the isothermal expansion shown in Fig. 20-3?
A)104 J/K
B)63.1 J/K
C)45.2 J/K
D)90.8 J/K
E)The answer cannot be determined without knowing the temperature of the gas.

What is the change in entropy of 10.0 moles of monatomic ideal gas with a molar specific heat 3R/2 that reversibly undergoes the isothermal expansion shown in Fig. 20-3?
A)104 J/K
B)63.1 J/K
C)45.2 J/K
D)90.8 J/K
E)The answer cannot be determined without knowing the temperature of the gas.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
The temperature inside a Carnot refrigerator placed in a kitchen at 22°C is 2.0°C. The heat extracted from refrigerator is 89 MJ/h. What power is needed to operate this refrigerator?
A)1.7 kW
B)1.8 kW
C)1.5 kW
D)1.9 kW
E)1.6 kW
A)1.7 kW
B)1.8 kW
C)1.5 kW
D)1.9 kW
E)1.6 kW

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
An 800-g block of ice at 0.00°C is resting in a large bath of water at 0.00°C insulated from the environment. After an entropy change of this system of 600 J/K, how much ice remains unmelted? The latent heat of fusion of water is 333 J/g.
A)579 g
B)492 g
C)764 g
D)308 g
E)221 g
A)579 g
B)492 g
C)764 g
D)308 g
E)221 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
A 3.00-kg block of silicon at 60.0°C is immersed in 6.00 kg of mercury at 20.0°C. What is the entropy increase of this system as it moves to equilibrium? The specific heat of silicon is 0.17 cal/(g∙K) and the specific heat of mercury is 0.033 cal/(g∙K).
A)8.8 × 10-1 J/K
B)4.8 J/K
C)6.9 J/K
D)5.2 × 10-2 J/K
E)-0.46 J/K
A)8.8 × 10-1 J/K
B)4.8 J/K
C)6.9 J/K
D)5.2 × 10-2 J/K
E)-0.46 J/K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
1.0 kg of steam at 100°C condenses to water at 100°C. What is the change in entropy in the process?
A)zero
B)6.1 × 103 J/K
C)3.0 × 103 J/K
D)-6.1 × 103 J/K
E)none of the above
A)zero
B)6.1 × 103 J/K
C)3.0 × 103 J/K
D)-6.1 × 103 J/K
E)none of the above

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
24.0 moles of a diatomic ideal gas with a molar specific heat 5R/2 is heated from 20.0°C to 100°C. What is the change in entropy of the gas?
A)-0.365 J/K
B)0.365 J/K
C)802 J/K
D)-19.9 J/K
E)120 J/K
A)-0.365 J/K
B)0.365 J/K
C)802 J/K
D)-19.9 J/K
E)120 J/K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
What is the change in entropy when 50 g of ice melt at 0°C?
A)14.7 cal/K
B)4.0 kcal/K
C)zero
D)-4.0 kcal/K
E)-14.7 cal/K
A)14.7 cal/K
B)4.0 kcal/K
C)zero
D)-4.0 kcal/K
E)-14.7 cal/K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
Calculate the total entropy change that occurs when 2.00 kg of lead at 40.0°C are placed in a very large quantity of water at 10.0°C. The specific heat of lead is 0.031 cal/(g∙K).
A)190 J/K
B)100 J/K
C)6.6 J/K
D)6.2 J/K
E)1.4 J/K
A)190 J/K
B)100 J/K
C)6.6 J/K
D)6.2 J/K
E)1.4 J/K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
Calculate the entropy change of the lead that occurs when 2.00 kg of lead at 40.0°C are placed in a very large quantity of water at 10.0°C. The specific heat of lead is 0.031 cal/(g∙K).
A)-12.6 J/K
B)86.0 J/K
C)-26.1 J/K
D)-86.0 J/K
E)-6.24 J/K
A)-12.6 J/K
B)86.0 J/K
C)-26.1 J/K
D)-86.0 J/K
E)-6.24 J/K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
The change in entropy of 20.0 moles of ideal monatomic constant volume gas as it changes from an initial temperature 300 K to its final temperature is 200 J/K. What is the final temperature of the gas?
A)669 K
B)562 K
C)427 K
D)187 K
E)345 K
A)669 K
B)562 K
C)427 K
D)187 K
E)345 K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
A system consists of two thermal reservoirs in contact with each other, one at temperature 300°C and the other at temperature 200°C. 600 J of heat transfers from the 300°C reservoir to the 200°C reservoir. What is the change in entropy of this system?
A)0.221 J/K
B)1.00 J/K
C)5.00 J/K
D)-1.00 J/K
E)2.31 J/K
A)0.221 J/K
B)1.00 J/K
C)5.00 J/K
D)-1.00 J/K
E)2.31 J/K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
A refrigerator removes heat from the freezing compartment at the rate of 20 kJ and ejects 24 kJ into a room per cycle. How much work is required in each cycle?
A)4 kJ
B)20 kJ
C)22 kJ
D)24 kJ
E)44 kJ
A)4 kJ
B)20 kJ
C)22 kJ
D)24 kJ
E)44 kJ

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
What is the coefficient of performance of an ideal Carnot heat pump used to heat a house whose inside temperature is 24°C when the outdoor temperature is -12°C?
A)8.25
B)2.75
C)0.67
D)0.53
E)5.00
A)8.25
B)2.75
C)0.67
D)0.53
E)5.00

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
A 2.00-kg block of aluminum at 50.0 °C is dropped into 5.00 kg of water at 20.0 °C. What is the change in entropy during the approach to equilibrium, assuming no heat is exchanged with the environment? The specific heat of aluminum is 0.22 cal/(g∙K).
A)8.08 J/K
B)10.1 J/K
C)3.25 × 10-2 J/K
D)3.80 × 10-3 J/K
E)2.41 × 10-3 J/K
A)8.08 J/K
B)10.1 J/K
C)3.25 × 10-2 J/K
D)3.80 × 10-3 J/K
E)2.41 × 10-3 J/K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
During each cycle of operation, a refrigerator absorbs 230 J of heat from the freezer and expels 356 J of heat to the room. How much work input is required in each cycle?
A)712 J
B)586 J
C)460 J
D)310 J
E)126 J
A)712 J
B)586 J
C)460 J
D)310 J
E)126 J

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
When 0.50 kg of ice freezes, the change in entropy is
A)zero.
B)365 J/K.
C)610 J/K.
D)-610 J/K.
E)none of the above
A)zero.
B)365 J/K.
C)610 J/K.
D)-610 J/K.
E)none of the above

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
A gas consisting of 20.0 moles of monatomic ideal gas has a change in entropy of 200 J/K when taken through a process that starts at with a volume of 6.00 m3 at 300 K and ends with a volume 2.00 m3. The molar specific heat of the gas is 3R/2 for constant volume processes. What is the final temperature of the gas?
A)680 K
B)2780 K
C)1860 K
D)100 K
E)1390 K
A)680 K
B)2780 K
C)1860 K
D)100 K
E)1390 K

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


