Deck 4: Chemical Composition
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/111
Play
Full screen (f)
Deck 4: Chemical Composition
1
A 6.25 g sample of magnetite (Fe3O4) contains 4.52 g of Fe.What are the percentages of iron and oxygen in magnetite?
A)28.6% Fe and 71.4% O
B)42.8% Fe and 57.2% O
C)72.3% Fe and 27.7% O
D)27.7% Fe and 72.3% O
E)57.2% Fe and 42.8% O
A)28.6% Fe and 71.4% O
B)42.8% Fe and 57.2% O
C)72.3% Fe and 27.7% O
D)27.7% Fe and 72.3% O
E)57.2% Fe and 42.8% O
72.3% Fe and 27.7% O
2
A 5.05 g sample of quartz (SiO2) contains 2.36 g of silicon.What are the percentages of silicon and oxygen in quartz?
A)53.3% Si and 46.7% O
B)46.7% Si and 53.3% O
C)29.9% Si and 70.1% O
D)70.1% Si and 29.9% O
E)46.7% Si, and insufficient information to calculate % O
A)53.3% Si and 46.7% O
B)46.7% Si and 53.3% O
C)29.9% Si and 70.1% O
D)70.1% Si and 29.9% O
E)46.7% Si, and insufficient information to calculate % O
46.7% Si and 53.3% O
3
How many nitrate ions are present in 0.200 mol of Zn(NO3)2?
A)1.20 x 1023 NO3- ions
B)2.41 x 1023 NO3- ions
C)3.01 x 1024 NO3- ions
D)6.64 x 10-25 NO3- ions
E)0.400 NO3- ions
A)1.20 x 1023 NO3- ions
B)2.41 x 1023 NO3- ions
C)3.01 x 1024 NO3- ions
D)6.64 x 10-25 NO3- ions
E)0.400 NO3- ions
2.41 x 1023 NO3- ions
4
How many formula units are in 2.0 moles of Fe(NO3)2?
A)1.2 x 1024
B)3.6 x 1024
C)1.1 x 1025
D)2.0
E)18
A)1.2 x 1024
B)3.6 x 1024
C)1.1 x 1025
D)2.0
E)18

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
5
How many formula units are there in 2.5 moles of MgCl2?
A)2.5
B)7.5
C)1.5 x 1024
D)4.5 x 1024
E)4.2 x 10-24
A)2.5
B)7.5
C)1.5 x 1024
D)4.5 x 1024
E)4.2 x 10-24

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
6
How many molecules of CO2 are present in 0.100 mol of CO2?
A)6.02 x 1022 molecules
B)4.40 molecules
C)1.66 x 10-25 molecules
D)6.02 x 1024 molecules
E)0.100 molecules
A)6.02 x 1022 molecules
B)4.40 molecules
C)1.66 x 10-25 molecules
D)6.02 x 1024 molecules
E)0.100 molecules

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following sets of formulas is correct for the molecules in the figure? 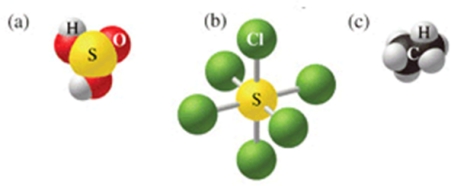
A)H2SO3, SCl5, C2H5
B)H2SO4, SCl5, C2H5
C)H2SO4, SCl6, C2H6
D)H2SO4, SCl6, CH6
E)H2SO3, SCl6, C2H6

A)H2SO3, SCl5, C2H5
B)H2SO4, SCl5, C2H5
C)H2SO4, SCl6, C2H6
D)H2SO4, SCl6, CH6
E)H2SO3, SCl6, C2H6

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following sets of formulas is correct for the molecules in the figure? 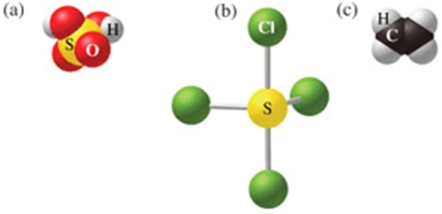
A)H2SO3, SCl4, C2H4
B)H2SO3, SCl3, C2H4
C)H2SO4, SCl3, C2H4
D)H2SO4, SCl4, CH4
E)H2SO4, SCl4, C2H4

A)H2SO3, SCl4, C2H4
B)H2SO3, SCl3, C2H4
C)H2SO4, SCl3, C2H4
D)H2SO4, SCl4, CH4
E)H2SO4, SCl4, C2H4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
9
How many oxygen atoms are there in 0.25 mole of CO2?
A)0.50
B)0.25
C)3.0 x 1023
D)1.5 x 1023
E)4.2 x 10-25
A)0.50
B)0.25
C)3.0 x 1023
D)1.5 x 1023
E)4.2 x 10-25

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
10
Barium sulfate is a compound used to assist in diagnosing medical problems through x-ray analysis and is 58.8% barium.What mass of barium is present in a 620 mg tablet of barium sulfate?
A)81 mg
B)230 mg
C)360 mg
D)1100 mg
E)11 mg
A)81 mg
B)230 mg
C)360 mg
D)1100 mg
E)11 mg

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
11
How many molecules of HCl are present in 0.250 mol of HCl?
A)4.15 x 10-25 molecules
B)1.51 x 1023 molecules
C)2.41 x 1024 molecules
D)9.11 molecules
E)0.250 molecules
A)4.15 x 10-25 molecules
B)1.51 x 1023 molecules
C)2.41 x 1024 molecules
D)9.11 molecules
E)0.250 molecules

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
12
How many carbon atoms are there in 0.50 mole of CO2?
A)0.50
B)1.50
C)3.0 x 1023
D)9.0 x 1023
E)8.3 x 1025
A)0.50
B)1.50
C)3.0 x 1023
D)9.0 x 1023
E)8.3 x 1025

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is incorrect?
A)H2SO3 has 1.5 times as many oxygen atoms as hydrogen atoms.
B)Fe2(SO4)3 has six times as many oxygen atoms as iron ions.
C)N2H4 has twice as many hydrogen atoms as nitrogen atoms.
D)MgF2 has half as many fluoride ions as magnesium ions.
E)Carbon dioxide has twice as many oxygen atoms as carbon atoms.
A)H2SO3 has 1.5 times as many oxygen atoms as hydrogen atoms.
B)Fe2(SO4)3 has six times as many oxygen atoms as iron ions.
C)N2H4 has twice as many hydrogen atoms as nitrogen atoms.
D)MgF2 has half as many fluoride ions as magnesium ions.
E)Carbon dioxide has twice as many oxygen atoms as carbon atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements is incorrect?
A)CH4 has ¼ as many carbon atoms as hydrogen atoms.
B)H2O has twice as many oxygen atoms as hydrogen atoms.
C)SO3 has three times as many oxygen atoms as sulfur atoms.
D)P4O10 has 2.5 times as many oxygen atoms as phosphorus atoms.
E)SrF2 has twice as many fluoride ions as strontium ions.
A)CH4 has ¼ as many carbon atoms as hydrogen atoms.
B)H2O has twice as many oxygen atoms as hydrogen atoms.
C)SO3 has three times as many oxygen atoms as sulfur atoms.
D)P4O10 has 2.5 times as many oxygen atoms as phosphorus atoms.
E)SrF2 has twice as many fluoride ions as strontium ions.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
15
4.Malachite is a green colored mineral that is 57.5% copper.What mass of copper is present in a 250.0 g sample of malachite?
A)63.6 g
B)127 g
C)435 g
D)36.5 g
E)144 g
A)63.6 g
B)127 g
C)435 g
D)36.5 g
E)144 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
16
A 7.50 g sample of pyrite (FeS2) contains 3.49 g of iron.What are the percentages of iron and sulfur in pyrite (also known as "Fool's Gold" because of its golden color)?
A)57.4% Fe and 42.6% S
B)66.7% Fe and 33.3% S
C)33.3% Fe and 66.7% S
D)46.5% Fe and 53.5% S
E)53.5% Fe and 46.5% S
A)57.4% Fe and 42.6% S
B)66.7% Fe and 33.3% S
C)33.3% Fe and 66.7% S
D)46.5% Fe and 53.5% S
E)53.5% Fe and 46.5% S

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is incorrect?
A)N2H4 has four times as many hydrogen atoms as nitrogen atoms.
B)SF4 has ¼ as many sulfur atoms as fluorine atoms.
C)SO2 has twice as many oxygen atoms as sulfur atoms.
D)Ca(NO3)2 has six times as many oxygen atoms as calcium ions.
E)H2SO4 has twice as many oxygen atoms as hydrogen atoms.
A)N2H4 has four times as many hydrogen atoms as nitrogen atoms.
B)SF4 has ¼ as many sulfur atoms as fluorine atoms.
C)SO2 has twice as many oxygen atoms as sulfur atoms.
D)Ca(NO3)2 has six times as many oxygen atoms as calcium ions.
E)H2SO4 has twice as many oxygen atoms as hydrogen atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
18
How many chloride ions are present in 0.100 mol of MgCl2?
A)0.200 Cl- ions
B)6.02 x 1022 Cl- ions
C)1.20 x 1023 Cl- ions
D)3.32 x 10-25 Cl- ions
E)3.01 x 1024 Cl- ions
A)0.200 Cl- ions
B)6.02 x 1022 Cl- ions
C)1.20 x 1023 Cl- ions
D)3.32 x 10-25 Cl- ions
E)3.01 x 1024 Cl- ions

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
19
How many formula units are in 0.25 mole of Na2O?
A)4.5 x 1023
B)0.75
C)1.5 x 1023
D)4.2 x 10-25
E)0.25
A)4.5 x 1023
B)0.75
C)1.5 x 1023
D)4.2 x 10-25
E)0.25

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements is incorrect?
A)PH3 has 3 times as many hydrogen atoms as phosphorus atoms.
B)MgO has an equal number of magnesium ions and oxygen ions.
C)BaBr2 has half as many barium ions as bromide ions.
D)N2O5 has 2.5 times as many nitrogen atoms as oxygen atoms.
E)SF4 has 4 times as many fluorine atoms as sulfur atoms.
A)PH3 has 3 times as many hydrogen atoms as phosphorus atoms.
B)MgO has an equal number of magnesium ions and oxygen ions.
C)BaBr2 has half as many barium ions as bromide ions.
D)N2O5 has 2.5 times as many nitrogen atoms as oxygen atoms.
E)SF4 has 4 times as many fluorine atoms as sulfur atoms.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
21
If 4.05 x 1023 molecules of a substance have a mass of 86.2 g, what is the molar mass of the substance?
A)128 g/mol
B)3.49 x 1025 g/mol
C)2.13 x 1022 g/mol
D)4.70 x 1021 g/mol
E)58.0 g/mol
A)128 g/mol
B)3.49 x 1025 g/mol
C)2.13 x 1022 g/mol
D)4.70 x 1021 g/mol
E)58.0 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
22
How many hydrogen atoms are there in 2.0 moles of CH4?
A)8.0
B)2.0
C)1.2 x 1024
D)1.3 x 10-23
E)4.8 x 1024
A)8.0
B)2.0
C)1.2 x 1024
D)1.3 x 10-23
E)4.8 x 1024

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
23
Rank the following in order of increasing mass: 1.0 mole of methane (CH4), 0.50 mole of water (H2O), 0.20 mole of Fe, and 0.010 mole of U.
A)U < Fe < H2O < CH4
B)H2O < CH4 < Fe < U
C)H2O < CH4 < U < Fe
D)U < H2O < CH4 < Fe
E)U < H2O < Fe < CH4
A)U < Fe < H2O < CH4
B)H2O < CH4 < Fe < U
C)H2O < CH4 < U < Fe
D)U < H2O < CH4 < Fe
E)U < H2O < Fe < CH4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the molar mass of CuSO4.5H2O.
A)159.62 g/mol
B)249.70 g/mol
C)177.64 g/mol
D)185.72 g/mol
E)446.48 g/mol
A)159.62 g/mol
B)249.70 g/mol
C)177.64 g/mol
D)185.72 g/mol
E)446.48 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements regarding atoms, molecules, and moles is correct?
A)Chemists are inherently lazy, so they weigh substances in order to avoid counting out the atoms or molecules in a sample.
B)It would be possible for an individual to count out a mole of atoms or molecules if they had a few days to do it.
C)A single grain of sand has about as many formula units of SiO2 as there are sand grains on all of the beaches on Earth.
D)A mole of HCl would have the same mass as a mole of NaCl, since they have the same number of particles.
E)Since a mole of LiCl has a mass of 42.39 g, the average mass of a LiCl formula unit would be 42.39 mole.
A)Chemists are inherently lazy, so they weigh substances in order to avoid counting out the atoms or molecules in a sample.
B)It would be possible for an individual to count out a mole of atoms or molecules if they had a few days to do it.
C)A single grain of sand has about as many formula units of SiO2 as there are sand grains on all of the beaches on Earth.
D)A mole of HCl would have the same mass as a mole of NaCl, since they have the same number of particles.
E)Since a mole of LiCl has a mass of 42.39 g, the average mass of a LiCl formula unit would be 42.39 mole.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
26
Rank the substances in the figure from least atoms per mole to most atoms per mole. 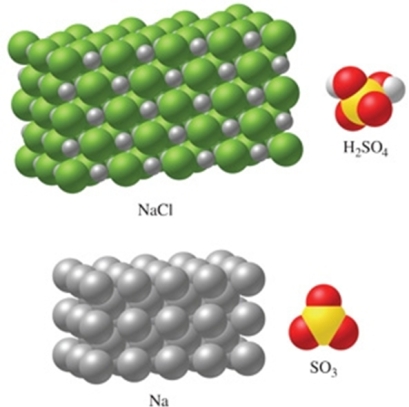
A)SO3 < H2SO4 < NaCl < Na
B)Na < NaCl < SO3 < H2SO4
C)Na < NaCl < H2SO4 < SO3
D)NaCl < Na < SO3 < H2SO4
E)H2SO4 < SO3 < NaCl < Na

A)SO3 < H2SO4 < NaCl < Na
B)Na < NaCl < SO3 < H2SO4
C)Na < NaCl < H2SO4 < SO3
D)NaCl < Na < SO3 < H2SO4
E)H2SO4 < SO3 < NaCl < Na

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
27
How many oxygen atoms are present in 1.50 mol of Zn(NO3)2?
A)4.50 atoms
B)9.00 atoms
C)9.03 x 1023 atoms
D)5.42 x 1024 atoms
E)1.49 x 10-23 atoms
A)4.50 atoms
B)9.00 atoms
C)9.03 x 1023 atoms
D)5.42 x 1024 atoms
E)1.49 x 10-23 atoms

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
28
Rank the following in order of increasing mass: 1.0 mole of SF4, 0.50 mole of H2S, 0.20 mole of Cu, and 0.10 mole of Pb.
A)Pb < Cu < H2S < SF4
B)Cu < H2S < Pb < SF4
C)Pb < Cu < SF4 < H2S
D)Cu < H2S < SF4 < Pb
E)SF4 < H2S < Cu < Pb
A)Pb < Cu < H2S < SF4
B)Cu < H2S < Pb < SF4
C)Pb < Cu < SF4 < H2S
D)Cu < H2S < SF4 < Pb
E)SF4 < H2S < Cu < Pb

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the molar mass of PH3.
A)40.11 g/mol
B)31.98 g/mol
C)33.99 g/mol
D)93.92 g/mol
E)2.05 x 1025 g/mol
A)40.11 g/mol
B)31.98 g/mol
C)33.99 g/mol
D)93.92 g/mol
E)2.05 x 1025 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate the molar mass of HCl.
A)36.46 g/mol
B)13.02 g/mol
C)2.196 x 1025 g/mol
D)6.054 x 10-23 g/mol
E)72.92 g/mol
A)36.46 g/mol
B)13.02 g/mol
C)2.196 x 1025 g/mol
D)6.054 x 10-23 g/mol
E)72.92 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
31
Rank the substances in the figure from least atoms per mole to most atoms per mole. 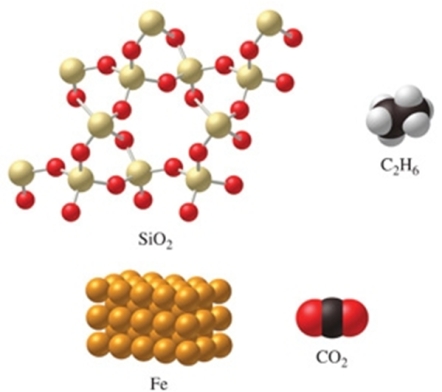
A)SiO2 < C2H6 < CO2 < Fe
B)C2H6 < SiO2 = CO2 < Fe
C)Fe < CO2 = SiO2 < C2H6
D)Fe < CO2 < SiO2 < C2H6
E)C2H6 < SiO2 < CO2 < Fe

A)SiO2 < C2H6 < CO2 < Fe
B)C2H6 < SiO2 = CO2 < Fe
C)Fe < CO2 = SiO2 < C2H6
D)Fe < CO2 < SiO2 < C2H6
E)C2H6 < SiO2 < CO2 < Fe

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
32
How many nitrogen atoms are present in 0.150 mol of Zn(NO3)2?
A)9.03 x 1022 atoms
B)1.81 x 1023 atoms
C)4.98 x 10-25 atoms
D)0.300 atoms
E)0.450 atoms
A)9.03 x 1022 atoms
B)1.81 x 1023 atoms
C)4.98 x 10-25 atoms
D)0.300 atoms
E)0.450 atoms

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
33
Calcium phosphate, Ca3(PO4)2, is used to treat calcium deficiencies.What is the molar mass of this compound?
A)87.05 g/mol
B)167.05 g/mol
C)279.21 g/mol
D)230.02 g/mol
E)310.18 g/mol
A)87.05 g/mol
B)167.05 g/mol
C)279.21 g/mol
D)230.02 g/mol
E)310.18 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the molar mass of C3H6Cl2.
A)155.08 g/mol
B)77.54 g/mol
C)48.47 g/mol
D)72.49 g/mol
E)112.98 g/mol
A)155.08 g/mol
B)77.54 g/mol
C)48.47 g/mol
D)72.49 g/mol
E)112.98 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
35
Rank the following in order of increasing mass: 2.0 mole of SO2, 1.0 mole of SO3, 0.50 mole of Mo, and 0.25 mole of Rn.
A)SO3 < SO2 < Rn < Mo
B)SO2 < SO3 < Mo < Rn
C)Mo < Rn < SO3 < SO2
D)Mo < Rn < SO2 < SO3
E)Rn < Mo < SO3 < SO2
A)SO3 < SO2 < Rn < Mo
B)SO2 < SO3 < Mo < Rn
C)Mo < Rn < SO3 < SO2
D)Mo < Rn < SO2 < SO3
E)Rn < Mo < SO3 < SO2

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
36
The formula for novocain, a local anesthetic, is C13H21N2O2.What is the molar mass of this compound?
A)43.03 g/mol
B)237.32 g/mol
C)2035.27 g/mol
D)38.00 g/mol
A)43.03 g/mol
B)237.32 g/mol
C)2035.27 g/mol
D)38.00 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
37
If 7.50 x 1024 molecules of a substance have a mass of 454 g, what is the molar mass of the substance?
A)2.73 x 1026 g/mol
B)3.41 x 1027 g/mol
C)5.65 x 103 g/mol
D)36.5 g/mol
E)12.5 g/mol
A)2.73 x 1026 g/mol
B)3.41 x 1027 g/mol
C)5.65 x 103 g/mol
D)36.5 g/mol
E)12.5 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the molar mass of Na2SO4.
A)94.05 g/mol
B)71.06 g/mol
C)119.06 g/mol
D)142.05 g/mol
E)110.05 g/mol
A)94.05 g/mol
B)71.06 g/mol
C)119.06 g/mol
D)142.05 g/mol
E)110.05 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the molar mass of Fe3(PO4)2.
A)237.64 g/mol
B)262.52 g/mol
C)245.79 g/mol
D)357.49 g/mol
E)525.04 g/mol
A)237.64 g/mol
B)262.52 g/mol
C)245.79 g/mol
D)357.49 g/mol
E)525.04 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
40
If 8.53 x 1024 molecules of a substance have a mass of 483 g, what is the molar mass of the substance?
A)6.86 x 103 g/mol
B)34.1 g/mol
C)4.12 x 1027 g/mol
D)1.77 x 1022 g/mol
E)0.0293 g/mol
A)6.86 x 103 g/mol
B)34.1 g/mol
C)4.12 x 1027 g/mol
D)1.77 x 1022 g/mol
E)0.0293 g/mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
41
Calculate the number of moles of NaHCO3 (sodium bicarbonate, or baking soda) in a 5.0 g sample of this substance.
A)0.096 mole
B)0.060 mole
C)420 moles
D)3.6 x 1022 moles
E)2.8 x 1023 mole
A)0.096 mole
B)0.060 mole
C)420 moles
D)3.6 x 1022 moles
E)2.8 x 1023 mole

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
42
If the molar mass of a substance is 20.0 g/mol, what is the mass of 3.01 x 1025 molecules of the substance?
A)0.400 g
B)10.0 g
C)1.00 x 103 g
D)6.02 x 1026 g
E)1.20 x 1025 g
A)0.400 g
B)10.0 g
C)1.00 x 103 g
D)6.02 x 1026 g
E)1.20 x 1025 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the number of moles of CaCO3 (calcium carbonate, or limestone) in a 20.0 g sample of this substance.
A)2.00 x 103 moles
B)0.200 mole
C)0.294 mole
D)1.36 x 103 moles
E)1.20 x 103 moles
A)2.00 x 103 moles
B)0.200 mole
C)0.294 mole
D)1.36 x 103 moles
E)1.20 x 103 moles

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
44
If the molar mass of a substance is 44.01 g/mol, what is the mass of 1.05 x 1024 molecules of the substance?
A)76.7 g
B)1.74 g
C)4.62 x 1025 g
D)4.19 x 10-23 g
E)25.2 g
A)76.7 g
B)1.74 g
C)4.62 x 1025 g
D)4.19 x 10-23 g
E)25.2 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
45
A chemical reaction produces 7.25 moles of barium sulfate, BaSO4.What mass of barium sulfate is produced?
A)185 g
B)233 g
C)1.69 x 103 g
D)31.1 g
E)3.11 x 10-2 g
A)185 g
B)233 g
C)1.69 x 103 g
D)31.1 g
E)3.11 x 10-2 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
46
If the molar mass of a substance is 20.0 g/mol, what is the mass of 4.01 x 1023 molecules of the substance?
A)30.0 g
B)13.3 g
C)8.02 x 1024 g
D)1.20 x 1025 g
E)3.00 g
A)30.0 g
B)13.3 g
C)8.02 x 1024 g
D)1.20 x 1025 g
E)3.00 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
47
Rank the following elements in order from least to most number of moles of atoms in a 10.0 g sample: Br, Fe, Pb, Hg
A)Br < Hg < Fe < Pb
B)Pb < Hg < Br < Fe
C)Fe < Br < Hg < Pb
D)Br < Fe < Hg < Pb
E)Hg < Pb < Br < Fe
A)Br < Hg < Fe < Pb
B)Pb < Hg < Br < Fe
C)Fe < Br < Hg < Pb
D)Br < Fe < Hg < Pb
E)Hg < Pb < Br < Fe

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
48
5.06 moles of iron(II) phosphate, Fe3(PO4)2, are produced in a reaction.What mass of iron(II) phosphate is produced?
A)520.g
B)20.3 g
C)1.81 x 103 g
D)3.05 x 1024 g
E)70.7 g
A)520.g
B)20.3 g
C)1.81 x 103 g
D)3.05 x 1024 g
E)70.7 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
49
How many molecules are in a sample of sucrose (sugar), C12H22O11 that has a mass of 15.0 g?
A)0.0438
B)2.64 x 1022
C)0.0824
D)4.96 x 1022
E)9.03 x 1024
A)0.0438
B)2.64 x 1022
C)0.0824
D)4.96 x 1022
E)9.03 x 1024

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
50
A chemical reaction requires 6.00 moles of Fe(NO3)3.What mass of iron(III) nitrate is needed?
A)24.3 g
B)875 g
C)40.3 g
D)1.45 x 103 g
E)515 g
A)24.3 g
B)875 g
C)40.3 g
D)1.45 x 103 g
E)515 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
51
Rank the following elements in order from least to most number of moles of atoms in a 10.0 g sample: Li, He, Mg, C
A)He < C < Li < Mg
B)C < Li < Mg < He
C)Li < C < Mg < He
D)He < Li < C < Mg
E)Mg < C < Li < He
A)He < C < Li < Mg
B)C < Li < Mg < He
C)Li < C < Mg < He
D)He < Li < C < Mg
E)Mg < C < Li < He

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
52
3.00 moles of NaOH (sodium hydroxide) are needed to prepare a solution.What mass of sodium hydroxide is required?
A)13.3 g
B)120.g
C)93 g
D)10.3 g
E)1.81 x 1024 g
A)13.3 g
B)120.g
C)93 g
D)10.3 g
E)1.81 x 1024 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
53
Rank the following elements in order from least to most number of moles of atoms in a 10.0 g sample: Sn, Si, Se, S
A)Sn < Se < S < Si
B)Si < S < Se < Sn
C)Si < S < Sn < Se
D)S < Si < Se < Sn
E)Se < Sn < S < Si
A)Sn < Se < S < Si
B)Si < S < Se < Sn
C)Si < S < Sn < Se
D)S < Si < Se < Sn
E)Se < Sn < S < Si

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
54
A 50.0 g sample of a compound contains 2.20 x 1023 molecules.Which of the following could be this compound?
A)PH3
B)NH3
C)PCl3
D)PCl5
E)NF3
A)PH3
B)NH3
C)PCl3
D)PCl5
E)NF3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
55
A 100.0 g sample of a compound contains 1.37 x 1024 molecules.Which of the following could be this compound?
A)CO2
B)NH3
C)CH4
D)CCl4
E)CF4
A)CO2
B)NH3
C)CH4
D)CCl4
E)CF4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
56
If the molar mass of a substance is 34.1 g/mol, what is the mass of 3.01 x 1024 molecules of the substance?
A)17.1 g
B)6.82 g
C)8.83 x 1022 g
D)1.03 x 1024 g
E)1.70 x 102 g
A)17.1 g
B)6.82 g
C)8.83 x 1022 g
D)1.03 x 1024 g
E)1.70 x 102 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
57
If you have 10.0 g of sodium chloride, NaCl, how many formula units are in the sodium chloride sample?
A)2.84 x 1025
B)3.52 x 1024
C)6.02 x 1024
D)1.03 x 1023
E)0.171
A)2.84 x 1025
B)3.52 x 1024
C)6.02 x 1024
D)1.03 x 1023
E)0.171

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
58
Calculate the number of moles of NaOH (sodium hydroxide, an ingredient in drain cleaners and oven cleaners) in a 10.0 g sample of this substance.
A)1.51 x 1023 moles
B)1.66 x 1023 mole
C)0.208 mole
D)4.00 x 102 moles
E)0.250 mole
A)1.51 x 1023 moles
B)1.66 x 1023 mole
C)0.208 mole
D)4.00 x 102 moles
E)0.250 mole

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
59
A chemical reaction requires 3.50 moles of copper(II) nitrate, Cu(NO3)2.What mass of copper(II) nitrate is needed?
A)327 g
B)93.6 g
C)188 g
D)656 g
E)53.6 g
A)327 g
B)93.6 g
C)188 g
D)656 g
E)53.6 g

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate the moles of sucrose, C12H22O11, in a 15.0-g sample of this substance.
A)342 mol
B)22.8 mol
C)5.13 x 103 mol
D)4.38 x 10-2 mol
E)0.517 mol
A)342 mol
B)22.8 mol
C)5.13 x 103 mol
D)4.38 x 10-2 mol
E)0.517 mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the substances in the figure have the same empirical and molecular formulas? 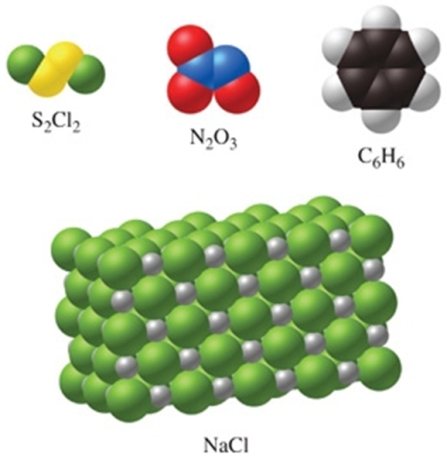
A)S2Cl2 and C6H6
B)none of the substances
C)all of the substances
D)N2O3 and NaCl
E)N2O3 only

A)S2Cl2 and C6H6
B)none of the substances
C)all of the substances
D)N2O3 and NaCl
E)N2O3 only

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
62
Methyl butyrate is a compound that is partially responsible for the flavor of apples.It consists of 58.80% C, 9.87% H, and 31.33% O.What is the empirical formula of methyl butyrate?
A)C6H10O3
B)C5H10O2
C)CH2O
D)C4.9H9.8O1.9
E)C4H9O2
A)C6H10O3
B)C5H10O2
C)CH2O
D)C4.9H9.8O1.9
E)C4H9O2

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
63
How many moles are in a sample of ethanol, CH3CH2OH that has a volume of 100.0 mL? The molar mass and density of ethanol are 46.07 g/mol and 0.789 g/mL, respectively.
A)78.9 mol
B)1.71 mol
C)36.3 mol
D)2.75 mol
E)0.504 mol
A)78.9 mol
B)1.71 mol
C)36.3 mol
D)2.75 mol
E)0.504 mol

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following statements regarding empirical formulas, molecular formulas, and percent composition is correct?
A)A compound with the molecular formula P4O10 would have the empirical formula P4O10.
B)It is not possible to determine the empirical formula of a compound if given only its percent composition.
C)If the molecular formula of a compound is C6H5Cl, its empirical formula is the same.
D)Empirical formulas contain more information than molecular formulas.
E)Formulas for ionic compounds are normally given as molecular formulas.
A)A compound with the molecular formula P4O10 would have the empirical formula P4O10.
B)It is not possible to determine the empirical formula of a compound if given only its percent composition.
C)If the molecular formula of a compound is C6H5Cl, its empirical formula is the same.
D)Empirical formulas contain more information than molecular formulas.
E)Formulas for ionic compounds are normally given as molecular formulas.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements regarding empirical formulas, molecular formulas, and percent composition is correct?
A)The empirical formula and molecular formula of a given compound are always the same.
B)Two compounds having different percent compositions of the same two elements are not the same compound.
C)The empirical formula and molecular formula of a given compound are never the same.
D)Data on the percent composition of the elements in a given compound is insufficient to determine the empirical formula of the compound.
E)The compound S2Cl2 would have the same empirical and molecular formulas.
A)The empirical formula and molecular formula of a given compound are always the same.
B)Two compounds having different percent compositions of the same two elements are not the same compound.
C)The empirical formula and molecular formula of a given compound are never the same.
D)Data on the percent composition of the elements in a given compound is insufficient to determine the empirical formula of the compound.
E)The compound S2Cl2 would have the same empirical and molecular formulas.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following statements regarding empirical formulas, molecular formulas, and percent composition is correct?
A)The compounds NO2 and N2O4 have the same empirical formula.
B)The compounds NO2 and N2O4 have the same molecular formula.
C)The empirical formula of H2O2 is H2O.
D)The empirical formula of N2O5 is NO2.5.
E)The compounds PCl3 and PCl5 would have the same percent composition.
A)The compounds NO2 and N2O4 have the same empirical formula.
B)The compounds NO2 and N2O4 have the same molecular formula.
C)The empirical formula of H2O2 is H2O.
D)The empirical formula of N2O5 is NO2.5.
E)The compounds PCl3 and PCl5 would have the same percent composition.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
67
Given the following molecular formulas, determine the empirical formula of each compound: Cl2O5, PbCl4, N2O4, C3H6Cl2.
A)ClO2.5, PbCl4, NO2, C3H6Cl2
B)Cl2O5, PbCl4, NO2, C3H6Cl2
C)Cl2O5, PbCl4, NO2, CH2Cl
D)Cl2O5, PbCl4, N2O4, C3H6Cl2
E)Cl2O5, PbCl, N2O4, C3H6Cl2
A)ClO2.5, PbCl4, NO2, C3H6Cl2
B)Cl2O5, PbCl4, NO2, C3H6Cl2
C)Cl2O5, PbCl4, NO2, CH2Cl
D)Cl2O5, PbCl4, N2O4, C3H6Cl2
E)Cl2O5, PbCl, N2O4, C3H6Cl2

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
68
Given the following molecular formulas, determine the empirical formula of each compound: N2O5, PCl3, H2O2, C6H4Cl2.
A)N2O5, PCl3, HO, C6H4Cl2
B)N2O5, PCl3, H2O, C6H4Cl2
C)N2O5, PCl3, H2O2, C3H2Cl2
D)NO2.5, PCl3, HO, C3H2Cl
E)N2O5, PCl3, HO, C3H2Cl
A)N2O5, PCl3, HO, C6H4Cl2
B)N2O5, PCl3, H2O, C6H4Cl2
C)N2O5, PCl3, H2O2, C3H2Cl2
D)NO2.5, PCl3, HO, C3H2Cl
E)N2O5, PCl3, HO, C3H2Cl

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
69
The molecular formula of the molecules in the figure is: 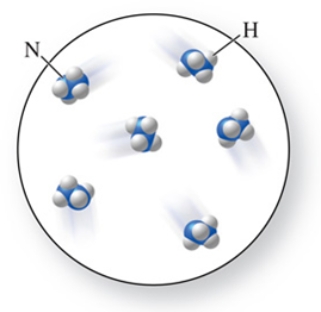
A)NH4
B)N2H4
C)NH3
D)N2H3
E)N4H2

A)NH4
B)N2H4
C)NH3
D)N2H3
E)N4H2

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
70
Putrescine is a compound that is partially responsible for the rotten smell of decaying flesh.It consists of 54.50% C, 13.73% H, and 31.77% N.What is the empirical formula of putrescine?
A)C5HN3
B)C4.5H13.5N2.3
C)C4H13N2
D)C2H6N
E)C4H12N2
A)C5HN3
B)C4.5H13.5N2.3
C)C4H13N2
D)C2H6N
E)C4H12N2

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
71
How many formula units are in a sample of rust, Fe2O3 that has a mass of 5.0 g?
A)1.9 x 1025
B)0.031
C)1.9 x 1022
D)0.070
E)4.2 x 1022
A)1.9 x 1025
B)0.031
C)1.9 x 1022
D)0.070
E)4.2 x 1022

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
72
Acetaminophen is the active ingredient in Tylenol.It consists of 63.56% C, 6.00% H, 9.27% N, and 21.17% O.What is the empirical formula of acetaminophen?
A)C8H8NO2
B)C8H9NO2
C)C6HNO2
D)C5.3H5.9N0.7O1.3
E)C4H4NO
A)C8H8NO2
B)C8H9NO2
C)C6HNO2
D)C5.3H5.9N0.7O1.3
E)C4H4NO

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
73
Rank the following compounds in order from least phosphorus atoms to most phosphorus atoms in a 50.0 g sample: P4, P4O10, PH3, PCl3
A)PCl3 < P4O10 < PH3 < P4
B)PCl3 < P4O10 < P4 < PH3
C)P4 < PH3 < P4O10 < PCl3
D)P4O10 < PCl3 < PH3 < P4
E)P4 < P4O10 < PCl3 < PH3
A)PCl3 < P4O10 < PH3 < P4
B)PCl3 < P4O10 < P4 < PH3
C)P4 < PH3 < P4O10 < PCl3
D)P4O10 < PCl3 < PH3 < P4
E)P4 < P4O10 < PCl3 < PH3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following statements regarding empirical and molecular formulas is correct?
A)Information on percent composition is all that is necessary to determine both an empirical and a molecular formula.
B)A compound with an empirical formula of CH2O and a molar mass of 90 g/mol would have a molecular formula of C3H6O3.
C)The compounds C2H4 and C3H6 have different empirical formulas.
D)The formula Ca2Cl4 is the correct empirical formula for calcium chloride.
E)The compound benzene has a molecular formula of C6H6, so its empirical formula would be C3H3.
A)Information on percent composition is all that is necessary to determine both an empirical and a molecular formula.
B)A compound with an empirical formula of CH2O and a molar mass of 90 g/mol would have a molecular formula of C3H6O3.
C)The compounds C2H4 and C3H6 have different empirical formulas.
D)The formula Ca2Cl4 is the correct empirical formula for calcium chloride.
E)The compound benzene has a molecular formula of C6H6, so its empirical formula would be C3H3.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the molecules in the figure have an empirical formula that is different from their molecular formula? 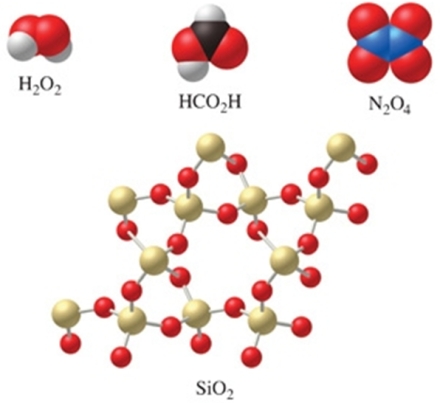
A)H2O2, N2O4, and HCO2H
B)SiO2
C)H2O2 and N2O4
D)all of the compounds
E)none of the compounds

A)H2O2, N2O4, and HCO2H
B)SiO2
C)H2O2 and N2O4
D)all of the compounds
E)none of the compounds

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
76
Acetic acid is the active ingredient in vinegar.It consists of 40.00% C, 6.714% H, and 53.29% O.What is the empirical formula of acetic acid?
A)C3.33H6.66O3.33
B)C3H6O3
C)CH2O
D)C2H4O2
E)CH3O
A)C3.33H6.66O3.33
B)C3H6O3
C)CH2O
D)C2H4O2
E)CH3O

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
77
Given the following molecular formulas, determine the empirical formula of each compound: P4O10, SnCl2, N2O3, CH3CO2H.
A)P2O5, SnCl2, N2O3, CH3CO2H
B)PO2.5, SnCl2, N2O3, CH3CO2H
C)P2O5, SnCl2, N2O3, CH2O
D)PO2.5, SnCl, NO1.5, CH3CO2H
E)P4O10, SnCl4, N2O3, CH2O
A)P2O5, SnCl2, N2O3, CH3CO2H
B)PO2.5, SnCl2, N2O3, CH3CO2H
C)P2O5, SnCl2, N2O3, CH2O
D)PO2.5, SnCl, NO1.5, CH3CO2H
E)P4O10, SnCl4, N2O3, CH2O

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
78
How many molecules are in a sample of water, H2O, which has a mass of 50.0 g?
A)1.67 x 1024 molecules
B)5.42 x 1026 molecules
C)2.16 x 1023 molecules
D)3.01 x 1025 molecules
E)8.30 x 10-23 molecules
A)1.67 x 1024 molecules
B)5.42 x 1026 molecules
C)2.16 x 1023 molecules
D)3.01 x 1025 molecules
E)8.30 x 10-23 molecules

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
79
Rank the following compounds in order from least nitrogen atoms to most nitrogen atoms in a 50.0 g sample: N2, N2O5, NH3, NH4Cl
A)N2 < N2O5 < NH3 < NH4Cl
B)N2O5 < NH4Cl < NH3 < N2
C)N2 < NH3 < NH4Cl < N2O5
D)NH3 < NH4Cl < N2 < N2O5
E)NH4Cl < NH3 < N2 < N2O5
A)N2 < N2O5 < NH3 < NH4Cl
B)N2O5 < NH4Cl < NH3 < N2
C)N2 < NH3 < NH4Cl < N2O5
D)NH3 < NH4Cl < N2 < N2O5
E)NH4Cl < NH3 < N2 < N2O5

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
80
Rank the following compounds in order from least chlorine atoms to most chlorine atoms in a 50.0 g sample: Cl2, ClF3, Cl2O, PCl3
A)ClF3 < Cl2O < Cl2 < PCl3
B)PCl3 < Cl2O < Cl2 < ClF3
C)ClF3 < PCl3 < Cl2O < Cl2
D)ClF3 < PCl3 < Cl2 < Cl2O
E)Cl2O < Cl2 < ClF3 < PCl3
A)ClF3 < Cl2O < Cl2 < PCl3
B)PCl3 < Cl2O < Cl2 < ClF3
C)ClF3 < PCl3 < Cl2O < Cl2
D)ClF3 < PCl3 < Cl2 < Cl2O
E)Cl2O < Cl2 < ClF3 < PCl3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck


