Deck 1: Matter and Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/110
Play
Full screen (f)
Deck 1: Matter and Energy
1
Which of these elements is a nonmetal?
A)Na
B)Mg
C)Cu
D)K
E)Cl
A)Na
B)Mg
C)Cu
D)K
E)Cl
Cl
2
Which of the following is not an example of matter?
A)air
B)light from a candle
C)wax
D)the propellant in an aerosol can
E)a stain on clothing
A)air
B)light from a candle
C)wax
D)the propellant in an aerosol can
E)a stain on clothing
light from a candle
3
 The symbol for the element barium is _________.
The symbol for the element barium is _________.A)B
B)Br
C)Ba
D)Be
E)Bi
Ba
4
Which of the following is not an example of matter?
A)a rock
B)a hot-air balloon
C)carbon dioxide in your exhaled breath
D)steam
E)heat from a barbeque grill
A)a rock
B)a hot-air balloon
C)carbon dioxide in your exhaled breath
D)steam
E)heat from a barbeque grill

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these substances is a compound?
A)Co
B)NI3
C)Fe
D)CCl4
E)both NI3 and CCl4
A)Co
B)NI3
C)Fe
D)CCl4
E)both NI3 and CCl4

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is an example of a pure substance?
A)a copper wire
B)milk
C)leather
D)a piece of carpet
E)ocean water
A)a copper wire
B)milk
C)leather
D)a piece of carpet
E)ocean water

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following does not apply to a chemical compound?
A)A chemical compound consists of two or more elements.
B)The elements in a compound are combined in definite proportions.
C)The characteristics of the compound are different from the characteristics of the elements from which it is made.
D)Compounds can be separated into their constituent elements using only physical methods.
E)A chemical compound can also be classified as a pure substance.
A)A chemical compound consists of two or more elements.
B)The elements in a compound are combined in definite proportions.
C)The characteristics of the compound are different from the characteristics of the elements from which it is made.
D)Compounds can be separated into their constituent elements using only physical methods.
E)A chemical compound can also be classified as a pure substance.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
8
The symbol for the element copper is __________.
A)Co
B)C
C)Cr
D)Cu
E)Ca
A)Co
B)C
C)Cr
D)Cu
E)Ca

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements regarding elements is incorrect?
A)Elements are the simplest building block of matter.
B)Elements cannot be broken down into simpler substances even by chemical means.
C)Some elements are not naturally occurring, and have been synthesized by scientists.
D)As the Greeks had thought, water is an element.
E)Elements are classified using a periodic table.
A)Elements are the simplest building block of matter.
B)Elements cannot be broken down into simpler substances even by chemical means.
C)Some elements are not naturally occurring, and have been synthesized by scientists.
D)As the Greeks had thought, water is an element.
E)Elements are classified using a periodic table.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
10
Which of these substances is an element?
A)C
B)CO
C)N2
D)HCl
E)both C and N2
A)C
B)CO
C)N2
D)HCl
E)both C and N2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
11
Which of these elements is a metal?
A)Ca
B)N
C)Ne
D)C
E)O
A)Ca
B)N
C)Ne
D)C
E)O

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
12
The symbol for the element potassium is__________.
A)P
B)Pt
C)K
D)Po
E)Pa
A)P
B)Pt
C)K
D)Po
E)Pa

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is an example of a pure substance?
A)sand
B)tap water
C)aluminum in a soda can (not considering the paint or plastic coatings)
D)river water
E)granite
A)sand
B)tap water
C)aluminum in a soda can (not considering the paint or plastic coatings)
D)river water
E)granite

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
14
Which image(s) in the figure represents a pure elemental substance? 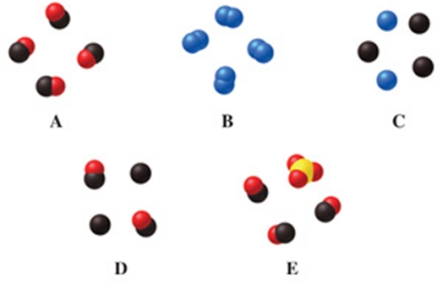
A)image A
B)image B
C)image C
D)images A and B
E)image E

A)image A
B)image B
C)image C
D)images A and B
E)image E

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is not an example of a mixture?
A)air
B)iced tea
C)24-carat gold
D)brass
E)a person
A)air
B)iced tea
C)24-carat gold
D)brass
E)a person

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
16
A combination of two or more substances that can be separated by using only a physical process is:
A)an element.
B)a compound.
C)a mixture.
D)a substance.
E)a composition.
A)an element.
B)a compound.
C)a mixture.
D)a substance.
E)a composition.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
17
Select the substance below which is a compound:
A)NO
B)Ir
C)Ni
D)Co
E)Rf
A)NO
B)Ir
C)Ni
D)Co
E)Rf

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
18
Which image(s) in the figure represents a mixture of two elements? 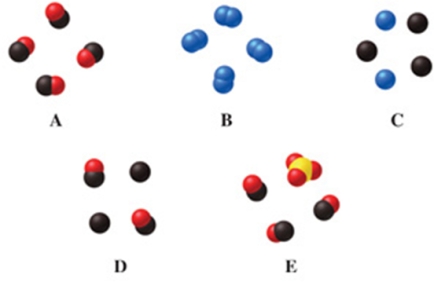
A)image A
B)image B
C)image C
D)image D
E)images C and D

A)image A
B)image B
C)image C
D)image D
E)images C and D

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is an example of matter?
A)sunlight
B)light from an incandescent bulb
C)helium in a balloon
D)heat from a car's radiator
E)all of these are correct
A)sunlight
B)light from an incandescent bulb
C)helium in a balloon
D)heat from a car's radiator
E)all of these are correct

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
20
Which image(s) in the figure represents a mixture of compounds? 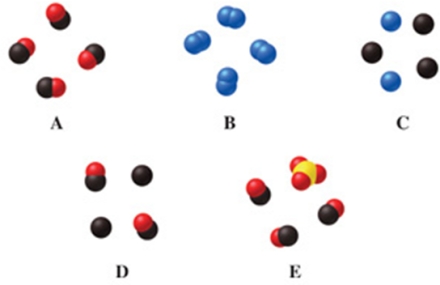
A)image A
B)images A and E
C)image C
D)image D
E)image E

A)image A
B)images A and E
C)image C
D)image D
E)image E

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements regarding the gaseous state of matter is incorrect?
A)The symbol for a gas is (g).
B)Gases consist of particles that are in constant random motion.
C)Gases can be compressed to smaller volumes.
D)It is possible for gases to mix together.
E)The particles in a gas are relatively close to one another.
A)The symbol for a gas is (g).
B)Gases consist of particles that are in constant random motion.
C)Gases can be compressed to smaller volumes.
D)It is possible for gases to mix together.
E)The particles in a gas are relatively close to one another.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
22
How many pounds of miniature candy bars are in a package that contains 197 g? (1 lb = 453.6 g)
A)0.0271 lb
B)0.434 lb
C)5.59 *103 lb
D)8.94 *105 lb
E)1.69 *10-3 lb
A)0.0271 lb
B)0.434 lb
C)5.59 *103 lb
D)8.94 *105 lb
E)1.69 *10-3 lb

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
23
A characteristic of a substance that involves the transformations the substance can undergo to produce a different substance is:
A)a physical property.
B)a chemical property.
C)a physical change.
D)a material property.
E)a characteristic property.
A)a physical property.
B)a chemical property.
C)a physical change.
D)a material property.
E)a characteristic property.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
24
A 1-ounce serving of Cheetos has 2.90 *102 mg of sodium.What is this mass in units of grams?
A)290,000 g
B)0.290 g
C)0.00345 g
D)3.45 g
E)29.0 g
A)290,000 g
B)0.290 g
C)0.00345 g
D)3.45 g
E)29.0 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
25
Consider the following transformation.Which of the statements best describes the process? 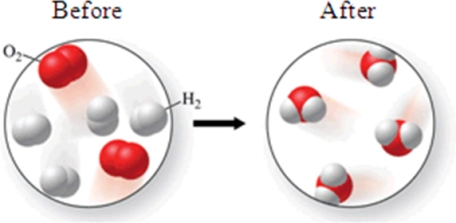
A)A physical change occurs in which no atoms rearrange.
B)A physical change occurs in which atoms rearrange to form a new compound.
C)A chemical change occurs in which no atoms rearrange.
D)A chemical change occurs in which atoms rearrange to form a new compound.
E)A chemical change occurs in which an ionic compound is formed from diatomic elements.

A)A physical change occurs in which no atoms rearrange.
B)A physical change occurs in which atoms rearrange to form a new compound.
C)A chemical change occurs in which no atoms rearrange.
D)A chemical change occurs in which atoms rearrange to form a new compound.
E)A chemical change occurs in which an ionic compound is formed from diatomic elements.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
26
The symbol Hg corresponds to which element?
A)magnesium
B)gallium
C)mercury
D)hydrogen
E)helium
A)magnesium
B)gallium
C)mercury
D)hydrogen
E)helium

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
27
A brownie contains 1.30 * 102 mg of sodium.What is this mass in units of grams?
A)1.30*105 g
B)0.130 g
C)13.0 g
D)7.69 g
E)0.00769 g
A)1.30*105 g
B)0.130 g
C)13.0 g
D)7.69 g
E)0.00769 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is the physical state of matter which does not have a characteristic shape, but takes on the shape of the filled part of its container?
A)solid
B)liquid
C)gas
D)liquid or gas
E)solid or liquid
A)solid
B)liquid
C)gas
D)liquid or gas
E)solid or liquid

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements is incorrect?
A)The burning of propane in a barbecue grill is a physical change.
B)The cooking of the meat on a barbecue grill is a chemical change.
C)Cleaning the grill afterwards using a steel brush is a physical change.
D)Further cleaning of the grill using a detergent is a chemical change.
E)Digestion of the meat by your body involves both physical and chemical changes.
A)The burning of propane in a barbecue grill is a physical change.
B)The cooking of the meat on a barbecue grill is a chemical change.
C)Cleaning the grill afterwards using a steel brush is a physical change.
D)Further cleaning of the grill using a detergent is a chemical change.
E)Digestion of the meat by your body involves both physical and chemical changes.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
30
Which physical state is represented in this image? 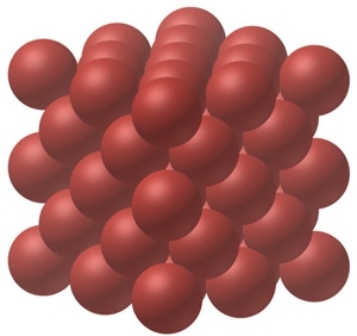
A)gas
B)liquid
C)solid
D)mixture

A)gas
B)liquid
C)solid
D)mixture

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
31
A 1-ounce serving of a breakfast cereal contains 1.60 *102 mg of potassium.What is this mass in units of grams?
A)0.160 g
B)1.60 *105 g
C)6.25 g
D)0.00625 g
E)16.0 g
A)0.160 g
B)1.60 *105 g
C)6.25 g
D)0.00625 g
E)16.0 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
32
The symbol for the element sodium is__________.
A)S
B)So
C)Sm
D)Na
E)Sn
A)S
B)So
C)Sm
D)Na
E)Sn

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
33
The symbol for the element iron is__________.
A)I
B)Ir
C)Fe
D)In
E)Ag
A)I
B)Ir
C)Fe
D)In
E)Ag

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements regarding the solid state of matter is incorrect?
A)The symbol for solid is (s).
B)Solids consist of particles that do not move past one another.
C)The particles in a solid are in close contact with one another.
D)Solids can be compressed to smaller volumes.
E)When a solid is heated, the particles begin to move faster.
A)The symbol for solid is (s).
B)Solids consist of particles that do not move past one another.
C)The particles in a solid are in close contact with one another.
D)Solids can be compressed to smaller volumes.
E)When a solid is heated, the particles begin to move faster.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
35
The symbol for the element calcium is__________.
A)Ca
B)C
C)Cm
D)Cu
E)Cl
A)Ca
B)C
C)Cm
D)Cu
E)Cl

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
36
Which physical state is represented in this image? 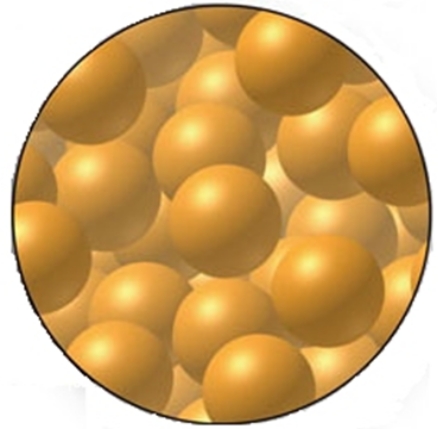
A)mixture
B)gas
C)liquid
D)solid

A)mixture
B)gas
C)liquid
D)solid

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
37
How many pounds of Spaghettios are in a can that contains 418 g? (1 lb = 453.6 g)
A)0.0576 lb
B)1.19 *104 lb
C)0.922 lb
D)1.90 *105 lb
E)3.60 *10-2 lb
A)0.0576 lb
B)1.19 *104 lb
C)0.922 lb
D)1.90 *105 lb
E)3.60 *10-2 lb

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements is incorrect?
A)The condensation of steam on a mirror is an example of a physical change.
B)The burning of a piece of charcoal to a white powder is an example of a physical change.
C)Evaporation of water from a fish tank is evidence of a physical change.
D)The fact that sulfur is a yellow powder is a physical property.
E)The fact that copper conducts electricity is a physical property.
A)The condensation of steam on a mirror is an example of a physical change.
B)The burning of a piece of charcoal to a white powder is an example of a physical change.
C)Evaporation of water from a fish tank is evidence of a physical change.
D)The fact that sulfur is a yellow powder is a physical property.
E)The fact that copper conducts electricity is a physical property.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
39
The symbol Au corresponds to which element?
A)arsenic
B)gold
C)mercury
D)silver
E)aluminum
A)arsenic
B)gold
C)mercury
D)silver
E)aluminum

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the following transformation.Which of the statements best describes the process? 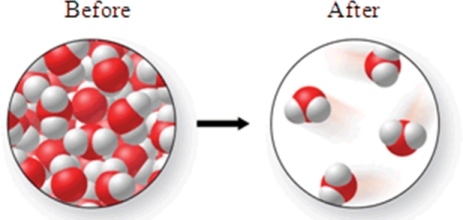
A)A chemical change occurs in which atoms rearrange to form a new compound.
B)A chemical change occurs in which an ionic compound is formed from diatomic elements.
C)A chemical change occurs in which no atoms rearrange to form a new substance.
D)A physical change occurs in which no atoms rearrange to form a new substance.
E)A physical change occurs in which atoms rearrange to form a new compound.

A)A chemical change occurs in which atoms rearrange to form a new compound.
B)A chemical change occurs in which an ionic compound is formed from diatomic elements.
C)A chemical change occurs in which no atoms rearrange to form a new substance.
D)A physical change occurs in which no atoms rearrange to form a new substance.
E)A physical change occurs in which atoms rearrange to form a new compound.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
41
A can of soup has a mass of 10.75 ounces.What is this mass in kg? (16 ounces = 453.6 g)
A)304.8 kg
B)0.3048 kg
C)2.370 *10-2 kg
D)1.481 *10-3 kg
E)4.876 *103 kg
A)304.8 kg
B)0.3048 kg
C)2.370 *10-2 kg
D)1.481 *10-3 kg
E)4.876 *103 kg

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
42
A can of cashews has a mass of 8.5 ounces.What is this mass in kg? (16 ounces = 453.6 g)
A)3900 kg
B)0.24 kg
C)240 kg
D)3.9 kg
E)0.14 kg
A)3900 kg
B)0.24 kg
C)240 kg
D)3.9 kg
E)0.14 kg

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
43
A bottle of fluoride rinse has a volume of 500 mL.Which of the following equivalences is incorrect?
A)V = 0.500 L
B)V = 500 cm3
C)V = 5.00 x 10 - 4 m3
D)V = 0.500 m3
E)both V = 500 cm3 and V = 0.500 m3
A)V = 0.500 L
B)V = 500 cm3
C)V = 5.00 x 10 - 4 m3
D)V = 0.500 m3
E)both V = 500 cm3 and V = 0.500 m3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
44
A box has dimensions of 2.5 cm by 3.0 cm by 4.0 cm.The volume of the box in milliliters and liters is:
A)30 mL and 0.030 L
B)30 mL and 30,000 L
C)7.5 mL and 0.0075 L
D)7.5 mL and 7,500 L
E)3,000 mL and 3.0 L
A)30 mL and 0.030 L
B)30 mL and 30,000 L
C)7.5 mL and 0.0075 L
D)7.5 mL and 7,500 L
E)3,000 mL and 3.0 L

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
45
A box has dimensions of 3.5 cm by 4.0 cm by 8.0 cm.The volume of the box in milliliters and liters is:
A)112 mL and 112,000 L
B)112 mL and 0.112 L
C)0.112 mL and 112 L
D)14 mL and 0.014 L
E)14 mL and 14,000 L
A)112 mL and 112,000 L
B)112 mL and 0.112 L
C)0.112 mL and 112 L
D)14 mL and 0.014 L
E)14 mL and 14,000 L

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
46
A bottle of Gatorade has a volume of 591 mL.What is this volume in fluid ounces? (1 fluid ounce = 29.57 mL)
A)20.0 fl oz
B)0.0500 fl oz
C)1.40 *105 fl oz
D)14.0 fl oz
E)35 fl oz
A)20.0 fl oz
B)0.0500 fl oz
C)1.40 *105 fl oz
D)14.0 fl oz
E)35 fl oz

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
47
The proprietor of a rock shop insists that a nugget is pure gold.If the nugget occupies a volume of 5.40 mL, what would its mass have to be if it were truly pure gold? (dgold = 19.3 g/mL)
A)104 g
B)3.57 g
C)0.279 g
D)13.9 g
E)insufficient information given
A)104 g
B)3.57 g
C)0.279 g
D)13.9 g
E)insufficient information given

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
48
A bottle of Gatorade has a volume of 591 mL.What is this volume in L?
A)5.91 L
B)591,000 L
C)0.00169 L
D)1.69 L
E)0.591 L
A)5.91 L
B)591,000 L
C)0.00169 L
D)1.69 L
E)0.591 L

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
49
A can of Spaghettios has a mass of 418 g.What is this mass in units of ounces? (16 ounces = 453.6 g)
A)0.922 oz
B)1.90 *105 oz
C)14.8 oz
D)1.19 *104 oz
E)5.76*-2 oz
A)0.922 oz
B)1.90 *105 oz
C)14.8 oz
D)1.19 *104 oz
E)5.76*-2 oz

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
50
A loaf of bread has a mass of 24 ounces.What is this mass in kg? (16 ounces = 453.6 g)
A)0.68 kg
B)680 kg
C)5.3*10-2 kg
D)0.85 kg
E)1.7 *105 kg
A)0.68 kg
B)680 kg
C)5.3*10-2 kg
D)0.85 kg
E)1.7 *105 kg

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
51
A box has dimensions of 4.0 cm by 8.5 cm by 2.0 cm.The volume of the box in mL is:
A)34 mL
B)0.068 mL
C)68 mL
D)17 mL
E)14.5 mL
A)34 mL
B)0.068 mL
C)68 mL
D)17 mL
E)14.5 mL

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
52
A bottle of soda has a volume of 474 mL.What is this volume in L?
A)4.74 L
B)0.474 L
C)47.4 L
D)2.11 L
E)0.00211 L
A)4.74 L
B)0.474 L
C)47.4 L
D)2.11 L
E)0.00211 L

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
53
A package of miniature candy bars has a mass of 197 g.What is this mass in units of ounces? (16 ounces = 453.6 g)
A)6.95 oz
B)0.434 oz
C)8.94*104 oz
D)1.43 *106 oz
E)2.71 *10-2 oz
A)6.95 oz
B)0.434 oz
C)8.94*104 oz
D)1.43 *106 oz
E)2.71 *10-2 oz

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
54
What is the mass in kilograms of a copper pipe that weighs 12.5 pounds? (1 lb = 453.6 g)
A)0.0276 kg
B)27.6 kg
C)5.67 *103 kg
D)5.67 kg
E)1.72 *10-3 kg
A)0.0276 kg
B)27.6 kg
C)5.67 *103 kg
D)5.67 kg
E)1.72 *10-3 kg

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
55
If the density of a certain alcohol is 0.785 g/mL, what volume of the alcohol would have a mass of 75.0 g?
A)0.955 mL
B)58.9 mL
C)75.8 mL
D)95.5 mL
E)insufficient information given
A)0.955 mL
B)58.9 mL
C)75.8 mL
D)95.5 mL
E)insufficient information given

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
56
A 1-ounce serving of Doritos has 17 g of carbohydrates.What is this mass in units of ounces? (16 ounces = 453.6 g)
A)2.7 oz
B)0.037 oz
C)0.60 oz
D)480 oz
E)28 oz
A)2.7 oz
B)0.037 oz
C)0.60 oz
D)480 oz
E)28 oz

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
57
The density of aluminum is 2.7 g/cm3.What is the mass of a piece of aluminum foil which is 10.0 cm by 5.0 cm by 0.0018 cm thick?
A)0.090 g
B)3.3 x 10 - 2 g
C)0.24 g
D)1.4 x 102 g
E)19 g
A)0.090 g
B)3.3 x 10 - 2 g
C)0.24 g
D)1.4 x 102 g
E)19 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
58
If the displacement (size) of a motorcycle engine is 1500 cm3, which of the following equivalences is incorrect?
A)V = 1500 mL
B)V = 1.500 L
C)V = 1.500 x 10 - 3 m3
D)V = 15.00 m3
E)both V = 1500 mL and V = 15.00 m3
A)V = 1500 mL
B)V = 1.500 L
C)V = 1.500 x 10 - 3 m3
D)V = 15.00 m3
E)both V = 1500 mL and V = 15.00 m3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
59
A bottle of soda has a volume of 474 mL.What is this volume in fluid ounces? (1 fluid ounce = 29.57 mL)
A)16.0 fl oz
B)0.0624 fl oz
C)1.75 *105 fl oz
D)17.5 fl oz
E)30 fl oz
A)16.0 fl oz
B)0.0624 fl oz
C)1.75 *105 fl oz
D)17.5 fl oz
E)30 fl oz

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
60
A typical light-weight bicycle weighs 17.5 pounds.What is the mass of a typical bike in kilograms? (1 lb = 453.6 g)
A)0.0386 kg
B)7.94 kg
C)7.94 * 106 kg
D)1.27 * 105 kg
E)2.41 *10-3 kg
A)0.0386 kg
B)7.94 kg
C)7.94 * 106 kg
D)1.27 * 105 kg
E)2.41 *10-3 kg

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
61
A diamond will float in water, but sink in carbon tetrachloride.Place these three substances in order from least density to greatest density.
A)water < diamond < carbon tetrachloride
B)diamond < water < carbon tetrachloride
C)carbon tetrachloride < diamond < water
D)diamond < carbon tetrachloride < water
E)water < carbon tetrachloride < diamond
A)water < diamond < carbon tetrachloride
B)diamond < water < carbon tetrachloride
C)carbon tetrachloride < diamond < water
D)diamond < carbon tetrachloride < water
E)water < carbon tetrachloride < diamond

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is not an example of a physical property?
A)The boiling point of acetone is 56 C.
B)Sand is more dense than water.
C)Helium is a gas at room temperature.
D)Copper gets a greenish coating on it when exposed to moist air.
E)Water is colorless.
A)The boiling point of acetone is 56 C.
B)Sand is more dense than water.
C)Helium is a gas at room temperature.
D)Copper gets a greenish coating on it when exposed to moist air.
E)Water is colorless.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is not an example of a chemical change?
A)Water becomes purple as Kool-Aid is dissolved in it.
B)Aluminum turns white after prolonged exposure to air.
C)A piece of charcoal becomes white after it burns.
D)Magnesium burns in air to make magnesium oxide.
E)Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas.
A)Water becomes purple as Kool-Aid is dissolved in it.
B)Aluminum turns white after prolonged exposure to air.
C)A piece of charcoal becomes white after it burns.
D)Magnesium burns in air to make magnesium oxide.
E)Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
64
If the temperature of water in a freezer decreases from 22 C to -25 C, what is the decrease in temperature in units of degrees Celsius and Kelvin?
A)47 C, 320 K
B)47 C, 273 K
C)47 C, 47 K
D)3 C, 276 K
E)3 C, 3 K
A)47 C, 320 K
B)47 C, 273 K
C)47 C, 47 K
D)3 C, 276 K
E)3 C, 3 K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is not an example of a physical property?
A)The boiling point of liquid nitrogen is 77 K.
B)Nitrogen is a gas at room temperature.
C)Nitrogen is colorless.
D)Nitrogen gas is less dense than oxygen gas.
E)Nitrogen combines with oxygen in an internal combustion engine to form oxides of nitrogen.
A)The boiling point of liquid nitrogen is 77 K.
B)Nitrogen is a gas at room temperature.
C)Nitrogen is colorless.
D)Nitrogen gas is less dense than oxygen gas.
E)Nitrogen combines with oxygen in an internal combustion engine to form oxides of nitrogen.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
66
If the density of a certain alcohol is 0.785 g/mL, what mass of the alcohol would have a volume of 200.0 mL?
A)2.55 g
B)157 g
C)200 g
D)255 g
E)3.92 x 10 - 3 g
A)2.55 g
B)157 g
C)200 g
D)255 g
E)3.92 x 10 - 3 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following statements is incorrect?
A)The scientific method is a way of looking at the world that is different from non-science forms of inquiry.
B)The scientific method does not allow for the use of inferences, and everything must be proved by direct observation.
C)A theory is a tentative explanation of the behavior or properties of matter.
D)Scientists must isolate and study one variable at a time when performing experiments.
E)A behavior of matter that has universal validity is called a law.
A)The scientific method is a way of looking at the world that is different from non-science forms of inquiry.
B)The scientific method does not allow for the use of inferences, and everything must be proved by direct observation.
C)A theory is a tentative explanation of the behavior or properties of matter.
D)Scientists must isolate and study one variable at a time when performing experiments.
E)A behavior of matter that has universal validity is called a law.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is not normally a part of scientific inquiry?
A)observations
B)philosophizing
C)theories
D)hypotheses
E)laws
A)observations
B)philosophizing
C)theories
D)hypotheses
E)laws

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is not a practice that would be employed by a scientist?
A)testing ideas by experimentation
B)organizing findings in specific ways
C)predicting the outcome of an experiment and then not testing the prediction
D)trying to explain why things happen
E)making physical models to explain the behavior of matter
A)testing ideas by experimentation
B)organizing findings in specific ways
C)predicting the outcome of an experiment and then not testing the prediction
D)trying to explain why things happen
E)making physical models to explain the behavior of matter

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following is not an example of a chemical property?
A)An iron nail will rust in water.
B)Sugar will dissolve in water.
C)A steak on a hot frying pan will turn brown.
D)Gasoline will burn if ignited.
E)Water can be decomposed to hydrogen and oxygen.
A)An iron nail will rust in water.
B)Sugar will dissolve in water.
C)A steak on a hot frying pan will turn brown.
D)Gasoline will burn if ignited.
E)Water can be decomposed to hydrogen and oxygen.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following statements is incorrect?
A)H2 molecules which are moving faster must have more kinetic energy than slower moving H2 molecules.
B)A book stored on a high bookshelf has potential energy.
C)A volleyball flying over a net has both kinetic energy and potential energy.
D)When gasoline is burned to power an engine, it releases only potential energy.
E)The water in a waterfall has kinetic, potential, and mechanical energy.
A)H2 molecules which are moving faster must have more kinetic energy than slower moving H2 molecules.
B)A book stored on a high bookshelf has potential energy.
C)A volleyball flying over a net has both kinetic energy and potential energy.
D)When gasoline is burned to power an engine, it releases only potential energy.
E)The water in a waterfall has kinetic, potential, and mechanical energy.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements is incorrect?
A)Energy is the capacity to do work or transfer heat.
B)Mechanical work occurs when a force acts over a distance.
C)Kinetic energy is the energy possessed by an object due to its position.
D)Potential energy can be possessed by chemical compounds.
E)A compound releases potential energy when it undergoes a spontaneous chemical reaction.
A)Energy is the capacity to do work or transfer heat.
B)Mechanical work occurs when a force acts over a distance.
C)Kinetic energy is the energy possessed by an object due to its position.
D)Potential energy can be possessed by chemical compounds.
E)A compound releases potential energy when it undergoes a spontaneous chemical reaction.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
73
A student exploring the desert finds a piece of metal with a volume of 29.9 cm3.If this metal has a mass of 337.5 g, which of the following is the metal most likely to be?
A)aluminum (density = 2.70 g/cm3)
B)sodium chloride (density = 2.16 g/cm3)
C)lead (density = 11.3 g/cm3)
D)gold (density = 19.3 g/cm3)
E)The student discovered a new metal with a density of 0.0886 g/cm3.
A)aluminum (density = 2.70 g/cm3)
B)sodium chloride (density = 2.16 g/cm3)
C)lead (density = 11.3 g/cm3)
D)gold (density = 19.3 g/cm3)
E)The student discovered a new metal with a density of 0.0886 g/cm3.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
74
An ice cube will sink in hexane, but float in water.Place these three substances in order from least density to greatest density.
A)ice < water < hexane
B)hexane < ice < water
C)hexane < water < ice
D)water < hexane < ice
E)water < ice < hexane
A)ice < water < hexane
B)hexane < ice < water
C)hexane < water < ice
D)water < hexane < ice
E)water < ice < hexane

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
75
If a child's balloon filled with helium were heated with a blow-dryer, the balloon would increase in volume.What would happen to the density of the helium in the balloon?
A)It would decrease.
B)It would increase.
C)It would remain the same.
D)A chemical reaction would occur, so it is impossible to predict.
E)The initial statement is incorrect-the volume of the balloon would not increase.
A)It would decrease.
B)It would increase.
C)It would remain the same.
D)A chemical reaction would occur, so it is impossible to predict.
E)The initial statement is incorrect-the volume of the balloon would not increase.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is not a form of energy?
A)chemical
B)mechanical
C)temperature
D)heat
E)electrical
A)chemical
B)mechanical
C)temperature
D)heat
E)electrical

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
77
The number 0.005925 correctly expressed in scientific notation is:
A)59.25 x 10 - 4
B)5.93 x 10 - 3
C)5.9 x 10 - 3
D)5.925 x 103
E)5.925 x 10 - 3
A)59.25 x 10 - 4
B)5.93 x 10 - 3
C)5.9 x 10 - 3
D)5.925 x 103
E)5.925 x 10 - 3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
78
If the temperature of a bowl of ice cream increases from - C to 25 C, what is the increase in temperature in units of degrees Celsius and Kelvin?
A)15 C, 288 K
B)35 C, 308 K
C)35 C, 273 K
D)35 C, 35 K
E)15 C, 273 K
A)15 C, 288 K
B)35 C, 308 K
C)35 C, 273 K
D)35 C, 35 K
E)15 C, 273 K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
79
A rubber stopper sinks in water, but floats in methylene chloride.Place these three substances in order from least density to greatest density.
A)rubber stopper < methylene chloride < water
B)rubber stopper < water < methylene chloride
C)water < methylene chloride < rubber stopper
D)water < rubber stopper < methylene chloride
E)methylene chloride < water < rubber stopper
A)rubber stopper < methylene chloride < water
B)rubber stopper < water < methylene chloride
C)water < methylene chloride < rubber stopper
D)water < rubber stopper < methylene chloride
E)methylene chloride < water < rubber stopper

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is not an example of a physical change?
A)Ice melts when warmed.
B)Dry ice sublimes (converts from a solid to a gas) at room temperature.
C)Liquid nitrogen converts to a gas at room temperature.
D)Blue copper sulfate crystals dissolve in water to form a blue solution.
E)When hydrogen and oxygen gas are mixed in the presence of a spark, water is formed.
A)Ice melts when warmed.
B)Dry ice sublimes (converts from a solid to a gas) at room temperature.
C)Liquid nitrogen converts to a gas at room temperature.
D)Blue copper sulfate crystals dissolve in water to form a blue solution.
E)When hydrogen and oxygen gas are mixed in the presence of a spark, water is formed.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck


