Deck 3: Chemical Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/112
Play
Full screen (f)
Deck 3: Chemical Compounds
1
The name of the oxoanion of sulfur with a 2-charge shown in the figure is: 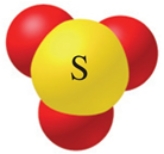
A)sulfite ion.
B)sulfate ion.
C)sulfide ion.
D)sulfurous ion.
E)sulfoxate ion.

A)sulfite ion.
B)sulfate ion.
C)sulfide ion.
D)sulfurous ion.
E)sulfoxate ion.
sulfite ion.
2
Which of the following statements regarding ionic and molecular compounds is correct?
A)Molecular compounds are expected to have very high melting and boiling points compared to ionic compounds.
B)Molecular compounds are strong electrolytes in aqueous solution.
C)An ionic compound can be a gas, liquid, or solid at room temperature.
D)Molecular compounds form crystalline solids that are hard and brittle.
E)Ionic compounds are expected to have very high melting and boiling points compared to ionic compounds.
A)Molecular compounds are expected to have very high melting and boiling points compared to ionic compounds.
B)Molecular compounds are strong electrolytes in aqueous solution.
C)An ionic compound can be a gas, liquid, or solid at room temperature.
D)Molecular compounds form crystalline solids that are hard and brittle.
E)Ionic compounds are expected to have very high melting and boiling points compared to ionic compounds.
Ionic compounds are expected to have very high melting and boiling points compared to ionic compounds.
3
Which of the following combinations of elements in a compound would be ionic?
A)barium and fluorine
B)hydrogen and sulfur
C)sulfur and oxygen
D)hydrogen and fluorine
E)all of these
A)barium and fluorine
B)hydrogen and sulfur
C)sulfur and oxygen
D)hydrogen and fluorine
E)all of these
barium and fluorine
4
The name of the oxoanion of nitrogen with a 1-charge shown in the figure is: 
A)nitrooxate ion.
B)nitride ion.
C)nitrite ion.
D)nitrate ion.
E)nitrous ion.

A)nitrooxate ion.
B)nitride ion.
C)nitrite ion.
D)nitrate ion.
E)nitrous ion.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the substance(s) shown in the figure is molecular? 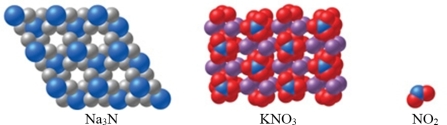
A)Na3N
B)KNO3
C)NO2
D)Both Na3N and KNO3
E)Both KNO3 and NO2

A)Na3N
B)KNO3
C)NO2
D)Both Na3N and KNO3
E)Both KNO3 and NO2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following combinations of elements in a compound would be molecular?
A)sodium and nitrogen
B)calcium and oxygen
C)nitrogen and oxygen
D)potassium and chlorine
E)sodium and sulfur
A)sodium and nitrogen
B)calcium and oxygen
C)nitrogen and oxygen
D)potassium and chlorine
E)sodium and sulfur

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
7
Rank the boiling points of the following compounds from lowest to highest: CO2, LiF, H2O
A)CO2 < H2O < LiF
B)CO2 < LiF < H2O
C)LiF < CO2 < H2O
D)H2O < LiF < CO2
E)H2O < CO2 < LiF
A)CO2 < H2O < LiF
B)CO2 < LiF < H2O
C)LiF < CO2 < H2O
D)H2O < LiF < CO2
E)H2O < CO2 < LiF

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is classified as an ionic compound?
A)NO2
B)CF4
C)CH3OH
D)CaCO3
E)all of these
A)NO2
B)CF4
C)CH3OH
D)CaCO3
E)all of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the substance(s) shown in the figure is ionic? 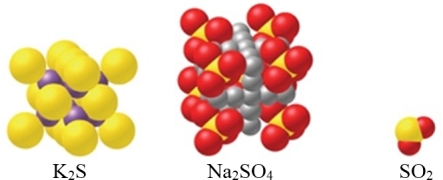
A)K2S
B)Na2SO4
C)SO2
D)Both Na2SO4 and SO2
E)Both K2S and Na2SO4

A)K2S
B)Na2SO4
C)SO2
D)Both Na2SO4 and SO2
E)Both K2S and Na2SO4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
10
Which combination of formula and name is incorrect?
A)S2- = sulfide ion
B)Br- = bromide ion
C)Li+ = lithium ion
D)Sr+ = strontium ion
E)P3- = phosphide ion
A)S2- = sulfide ion
B)Br- = bromide ion
C)Li+ = lithium ion
D)Sr+ = strontium ion
E)P3- = phosphide ion

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
11
Which combination of formula and name is incorrect?
A)Na+ = sodium ion
B)Ca2+ = calcium ion
C)N2- = nitride ion
D)F- = fluoride ion
E)O2-= oxide ion
A)Na+ = sodium ion
B)Ca2+ = calcium ion
C)N2- = nitride ion
D)F- = fluoride ion
E)O2-= oxide ion

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the correct formula for the nitride ion?
A)N2
B)NO2-
C)NO3-
D)N23-
E)N3-
A)N2
B)NO2-
C)NO3-
D)N23-
E)N3-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
13
The name of the oxoanion of nitrogen with a 1- charge shown in the figure is: 
A)nitrooxate ion.
B)nitride ion.
C)nitrite ion.
D)nitrate ion.
E)nitrous ion.

A)nitrooxate ion.
B)nitride ion.
C)nitrite ion.
D)nitrate ion.
E)nitrous ion.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
14
Rank the boiling points of the following compounds from lowest to highest: C12H22O11 (sucrose), O2, NaF
A)C12H22O11 (sucrose) < O2 < NaF
B)O2 < NaF < C12H22O11 (sucrose)
C)O2 < C12H22O11 (sucrose) < NaF
D)NaF < O2 < C12H22O11 (sucrose)
E)NaF < C12H22O11 (sucrose) < O2
A)C12H22O11 (sucrose) < O2 < NaF
B)O2 < NaF < C12H22O11 (sucrose)
C)O2 < C12H22O11 (sucrose) < NaF
D)NaF < O2 < C12H22O11 (sucrose)
E)NaF < C12H22O11 (sucrose) < O2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
15
Which combination of formula and name is incorrect?
A)K+ = potassium ion
B)I- = iodide ion
C)Mg+ = magnesium ion
D)S2- = sulfide ion
E)N3- = nitride ion
A)K+ = potassium ion
B)I- = iodide ion
C)Mg+ = magnesium ion
D)S2- = sulfide ion
E)N3- = nitride ion

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following combinations of elements in a compound would be ionic?
A)sodium and sulfur
B)nitrogen and sulfur
C)phosphorus and fluorine
D)hydrogen and oxygen
E)all of these
A)sodium and sulfur
B)nitrogen and sulfur
C)phosphorus and fluorine
D)hydrogen and oxygen
E)all of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is classified as a molecular compound?
A)OH-
B)N2
C)SO2
D)Mg(NO3)2
E)all of these
A)OH-
B)N2
C)SO2
D)Mg(NO3)2
E)all of these

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
18
The figure shows all but which of the following: 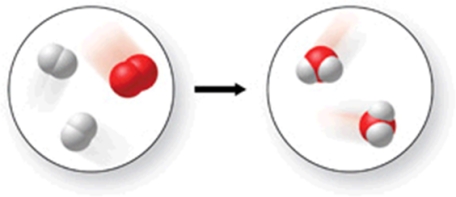
A)two elements and a compound.
B)a chemical reaction.
C)one mixture and one pure substance.
D)three compounds.
E)five molecules.

A)two elements and a compound.
B)a chemical reaction.
C)one mixture and one pure substance.
D)three compounds.
E)five molecules.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
19
Rank the boiling points of the following compounds from lowest to highest: N2, MgO, C2H5OH (ethyl alcohol)
A)MgO < C2H5OH (ethyl alcohol) < N2
B)MgO < N2 < C2H5OH (ethyl alcohol)
C)N2 < MgO < C2H5OH (ethyl alcohol)
D)N2 < C2H5OH (ethyl alcohol) < MgO
E)C2H5OH (ethyl alcohol) < N2 < MgO
A)MgO < C2H5OH (ethyl alcohol) < N2
B)MgO < N2 < C2H5OH (ethyl alcohol)
C)N2 < MgO < C2H5OH (ethyl alcohol)
D)N2 < C2H5OH (ethyl alcohol) < MgO
E)C2H5OH (ethyl alcohol) < N2 < MgO

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the correct formula for the bromide ion?
A)Br2
B)Br2-
C)Br22-
D)Br-
E)BrO3-
A)Br2
B)Br2-
C)Br22-
D)Br-
E)BrO3-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
21
Based on common charges, which formula for an ionic compound is incorrect?
A)NaClO3
B)Sr(NO3)2
C)AlCl3
D)KI
E)MgBr
A)NaClO3
B)Sr(NO3)2
C)AlCl3
D)KI
E)MgBr

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
22
The formula for the iodate ion is IO3-.What is the formula for the hypoiodite ion?
A)IO4-
B)IO2-
C)IO-
D)IO32-
E)I-
A)IO4-
B)IO2-
C)IO-
D)IO32-
E)I-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
23
The formula for the phosphate ion is:
A)PO32-
B)PO34-
C)PO33-
D)PO42-
E)PO43-
A)PO32-
B)PO34-
C)PO33-
D)PO42-
E)PO43-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following combinations of formula and name is incorrect?
A)hydroxide ion = OH-
B)nitrate ion = NO3-
C)sulfide ion = SO32-
D)nitrite ion = NO2-
E)sulfate ion = SO42-
A)hydroxide ion = OH-
B)nitrate ion = NO3-
C)sulfide ion = SO32-
D)nitrite ion = NO2-
E)sulfate ion = SO42-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following formulas for a compound containing the Cu2+ ion is incorrect?
A)Cu(NO3)2
B)CuS
C)CuN
D)CuSO4
E)CuSO3
A)Cu(NO3)2
B)CuS
C)CuN
D)CuSO4
E)CuSO3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
26
The formula for the bicarbonate ion is:
A)C2O32-
B)CO32-
C)HCO32-
D)HCO3 -
E)C2O42-
A)C2O32-
B)CO32-
C)HCO32-
D)HCO3 -
E)C2O42-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
27
The formula for the chlorate ion is ClO3-.What is the formula for the perchlorate ion?
A)ClO4-
B)ClO2-
C)ClO-
D)ClO32-
E)PClO3-
A)ClO4-
B)ClO2-
C)ClO-
D)ClO32-
E)PClO3-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following combinations of formula and name is incorrect?
A)carbonate ion = CO32-
B)ammonium ion = NH3
C)bicarbonate ion = HCO3-
D)periodate ion = IO4-
E)hypochlorite ion = ClO-
A)carbonate ion = CO32-
B)ammonium ion = NH3
C)bicarbonate ion = HCO3-
D)periodate ion = IO4-
E)hypochlorite ion = ClO-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following combinations of formula and name is incorrect?
A)nitride ion = NO2-
B)cyanide ion = CN-
C)chlorite ion = ClO2-
D)perchlorate ion = ClO4-
E)chloride ion = Cl-
A)nitride ion = NO2-
B)cyanide ion = CN-
C)chlorite ion = ClO2-
D)perchlorate ion = ClO4-
E)chloride ion = Cl-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
30
The formula for the bromate ion is BrO3-.What is the formula for the bromite ion?
A)BrO4-
B)BrO2-
C)BrO-
D)BrO32-
E)Br-
A)BrO4-
B)BrO2-
C)BrO-
D)BrO32-
E)Br-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
31
Based on common charges, which formula for an ionic compound is incorrect?
A)LiCO3
B)NaBr
C)CaO
D)SrS
E)MgCl2
A)LiCO3
B)NaBr
C)CaO
D)SrS
E)MgCl2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
32
Based on common charges, which formula for an ionic compound is incorrect?
A)Ba(NO3)2
B)Ca(OH)2
C)MgCl3
D)Al2O3
E)KBr
A)Ba(NO3)2
B)Ca(OH)2
C)MgCl3
D)Al2O3
E)KBr

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
33
The formula for the ammonium ion is:
A)NH3+
B)NH3
C)NH4+
D)NH4
E)NH4-
A)NH3+
B)NH3
C)NH4+
D)NH4
E)NH4-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
34
The formula for the chlorite ion is:
A)ClO-
B)ClO2-
C)ClO3-
D)ClO4-
E)ClO22-
A)ClO-
B)ClO2-
C)ClO3-
D)ClO4-
E)ClO22-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
35
The formula for the acetate ion is:
A)CH3CO2-
B)CH2CO3-
C)CH3CO22-
D)CH3CO23-
E)CH2CO2-
A)CH3CO2-
B)CH2CO3-
C)CH3CO22-
D)CH3CO23-
E)CH2CO2-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following formulas for a compound containing the Fe3+ ion is incorrect?
A)FeS
B)Fe2S3
C)FeN
D)FePO4
E)FeBr3
A)FeS
B)Fe2S3
C)FeN
D)FePO4
E)FeBr3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following formulas for a compound containing the Fe2+ ion is incorrect?
A)FeO
B)FeCl
C)FeS
D)FeSO4
E)Fe(NO3)2
A)FeO
B)FeCl
C)FeS
D)FeSO4
E)Fe(NO3)2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
38
Based on common charges, which formula for an ionic compound is incorrect?
A)Li2S
B)CaO
C)Na3N
D)K2Cl
E)Mg(OH)2
A)Li2S
B)CaO
C)Na3N
D)K2Cl
E)Mg(OH)2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
39
The formula for the permanganate ion is:
A)MnO4-
B)MnO22-
C)MnO3-
D)MnO32-
E)MnO42-
A)MnO4-
B)MnO22-
C)MnO3-
D)MnO32-
E)MnO42-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
40
Based on common charges, which formula for an ionic compound is incorrect?
A)CaCl
B)NaF
C)SrO
D)MgS
E)K3P
A)CaCl
B)NaF
C)SrO
D)MgS
E)K3P

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
41
What is the correct formula for the compound chromium(III) sulfide?
A)CrS
B)Cr3S
C)CrS3
D)Cr3S2
E)Cr2S3
A)CrS
B)Cr3S
C)CrS3
D)Cr3S2
E)Cr2S3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following formula/name pairs is incorrect?
A)FeO iron(II) oxide
B)K2SO4 potassium sulfide
C)MnO4 manganese(IV) oxide
D)LiOH lithium hydroxide
E)MgO magnesium oxide
A)FeO iron(II) oxide
B)K2SO4 potassium sulfide
C)MnO4 manganese(IV) oxide
D)LiOH lithium hydroxide
E)MgO magnesium oxide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
43
What is the correct formula for the compound lead(IV) oxide?
A)PbO2
B)PbO4
C)Pb2O
D)Pb4O
E)PbO
A)PbO2
B)PbO4
C)Pb2O
D)Pb4O
E)PbO

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following formula/name pairs is incorrect?
A)MnO manganese(II) oxide
B)CaO calcium oxide
C)MgCl2 magnesium chloride
D)AlP aluminum phosphide
E)Li2SO3 dilithium sulfite
A)MnO manganese(II) oxide
B)CaO calcium oxide
C)MgCl2 magnesium chloride
D)AlP aluminum phosphide
E)Li2SO3 dilithium sulfite

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following formula/name pairs is incorrect?
A)K2SO4 potassium sulfate
B)Na3N sodium nitride
C)Li2CO3 lithium carbonate
D)Fe2O3 iron(II) oxide
E)Cu2S copper(I) sulfide
A)K2SO4 potassium sulfate
B)Na3N sodium nitride
C)Li2CO3 lithium carbonate
D)Fe2O3 iron(II) oxide
E)Cu2S copper(I) sulfide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
46
The images in the figure represent aqueous solutions of ionic compounds.Which could correspond to Na2S? 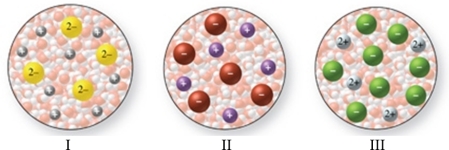
A)I only
B)II only
C)III only
D)I and III
E)II and III

A)I only
B)II only
C)III only
D)I and III
E)II and III

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
47
What is the formula for a compound composed of Cr3+ ions and SO32- ions?
A)CrSO3
B)Cr2(SO3)3
C)Cr3(SO3)2
D)Cr(SO3)2
E)Cr(SO3)3
A)CrSO3
B)Cr2(SO3)3
C)Cr3(SO3)2
D)Cr(SO3)2
E)Cr(SO3)3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
48
What is the formula for a compound composed of Ca2+ ions and PO43- ions?
A)CaPO4
B)Ca2(PO4)3
C)Ca3(PO4)2
D)Ca(PO4)2
E)Ca(PO4)3
A)CaPO4
B)Ca2(PO4)3
C)Ca3(PO4)2
D)Ca(PO4)2
E)Ca(PO4)3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
49
What is the correct formula for the compound formed between the cobalt(III) ion and the sulfate ion?
A)CoSO4
B)Co2(SO4)3
C)Co3(SO4)2
D)Co2S3
E)CoS3
A)CoSO4
B)Co2(SO4)3
C)Co3(SO4)2
D)Co2S3
E)CoS3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
50
What is the correct formula for the compound formed between iron(III) ion and the oxide ion?
A)FeO
B)Fe2O3
C)Fe3O2
D)Fe3O4
E)FeO3
A)FeO
B)Fe2O3
C)Fe3O2
D)Fe3O4
E)FeO3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
51
The images in the figure represent aqueous solutions of ionic compounds.Which could correspond to BaCl2? 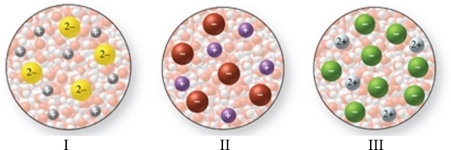
A)I only
B)II only
C)III only
D)I and III
E)II and III

A)I only
B)II only
C)III only
D)I and III
E)II and III

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following formula/name pairs is incorrect?
A)RbS rubidium sulfide
B)K2CO3 potassium carbonate
C)NaClO4 sodium chloroxate
D)Fe2S3 iron(III) sulfide
E)K2O potassium oxide
A)RbS rubidium sulfide
B)K2CO3 potassium carbonate
C)NaClO4 sodium chloroxate
D)Fe2S3 iron(III) sulfide
E)K2O potassium oxide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
53
What is the correct formula for the compound formed between copper(II) ion and the phosphide ion?
A)CuPO4
B)Cu2PO4
C)Cu3(PO4)2
D)Cu3P2
E)CuP
A)CuPO4
B)Cu2PO4
C)Cu3(PO4)2
D)Cu3P2
E)CuP

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
54
What is the correct formula for the compound formed between the aluminum ion and the sulfide ion?
A)Al3S2
B)AlS
C)Al2S
D)AlS2
E)Al2S3
A)Al3S2
B)AlS
C)Al2S
D)AlS2
E)Al2S3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is the correct formula for the compound with the name calcium nitride?
A)CaN
B)Ca3N2
C)Ca2N3
D)CaN2
E)Ca2N
A)CaN
B)Ca3N2
C)Ca2N3
D)CaN2
E)Ca2N

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
56
What is the correct formula for the compound formed between the calcium ion and the sulfate ion?
A)CaSO4
B)Ca2(SO4)2
C)Ca(SO4)2
D)CaS
E)CaSO3
A)CaSO4
B)Ca2(SO4)2
C)Ca(SO4)2
D)CaS
E)CaSO3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following formulas for a compound containing the Cr2+ ion is incorrect?
A)CrClO4
B)CrSO4
C)CrO
D)CrS
E)CrCl2
A)CrClO4
B)CrSO4
C)CrO
D)CrS
E)CrCl2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following formula/name pairs is incorrect?
A)BaBr2 barium bromide
B)CuO copper(II) oxide
C)MgCl2 magnesium dichloride
D)Sr(OH)2 strontium hydroxide
E)LiNO3 lithium nitrate
A)BaBr2 barium bromide
B)CuO copper(II) oxide
C)MgCl2 magnesium dichloride
D)Sr(OH)2 strontium hydroxide
E)LiNO3 lithium nitrate

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
59
What is the correct formula for the compound formed between the aluminum ion and the hydroxide ion?
A)AlOH
B)Al(OH)2
C)Al(OH)3
D)Al3OH
E)AlH3
A)AlOH
B)Al(OH)2
C)Al(OH)3
D)Al3OH
E)AlH3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is the correct formula for the compound with the name iron(II) phosphate?
A)FeP
B)Fe3(PO4)2
C)FePO4
D)Fe2(PO3)3
E)Fe2(PO4)3
A)FeP
B)Fe3(PO4)2
C)FePO4
D)Fe2(PO3)3
E)Fe2(PO4)3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
61
The compound NO2 is a reddish brown gas with a boiling of 21 C.Name this molecular compound.
A)nitrogen (IV) oxide
B)nitrogen dioxide
C)nitrogen oxide
D)nitrate
E)nitrite
A)nitrogen (IV) oxide
B)nitrogen dioxide
C)nitrogen oxide
D)nitrate
E)nitrite

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is the correct name for the compound Mn2O3?
A)dimanganese trioxide
B)manganate ion
C)manganese(III) oxide
D)manganese(II) oxide
E)manganese oxide
A)dimanganese trioxide
B)manganate ion
C)manganese(III) oxide
D)manganese(II) oxide
E)manganese oxide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is the correct formula for the compound with the name manganese(II) phosphate?
A)Mn3(PO4)2
B)Mn2(PO4)3
C)MnPO4
D)Mn3P2
E)Mn2P3
A)Mn3(PO4)2
B)Mn2(PO4)3
C)MnPO4
D)Mn3P2
E)Mn2P3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following compound names is not correct?
A)iron(III) sulfate
B)copper(II) diphosphide
C)sodium hydroxide
D)potassium nitrate
E)calcium sulfide
A)iron(III) sulfate
B)copper(II) diphosphide
C)sodium hydroxide
D)potassium nitrate
E)calcium sulfide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
65
The compound SO2 is a colorless gas with a boiling of -10 C.Name this molecular compound.
A)sulfate
B)sulfite
C)sulfur(IV) oxide
D)sulfur dioxide
E)sulfur(II) oxide
A)sulfate
B)sulfite
C)sulfur(IV) oxide
D)sulfur dioxide
E)sulfur(II) oxide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is the correct formula for the compound with the name copper(II) sulfate?
A)Cu2S
B)CuS2
C)Cu2SO4
D)CuSO4
E)CuSO3
A)Cu2S
B)CuS2
C)Cu2SO4
D)CuSO4
E)CuSO3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following compound names is not correct?
A)calcium hydroxide
B)iron(II) dichloride
C)potassium fluoride
D)sodium cyanide
E)ammonium hydroxide
A)calcium hydroxide
B)iron(II) dichloride
C)potassium fluoride
D)sodium cyanide
E)ammonium hydroxide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is the correct name for the compound CoBr2?
A)cobalt bromide
B)cobalt(II) bromide
C)cobalt dibromide
D)cobalt(II) bromate
E)cobalt(IV) bromide
A)cobalt bromide
B)cobalt(II) bromide
C)cobalt dibromide
D)cobalt(II) bromate
E)cobalt(IV) bromide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is the correct formula for the compound with the name aluminum sulfide?
A)AlS
B)Al3S2
C)Al2S3
D)AlSO4
E)Al2(SO4)3
A)AlS
B)Al3S2
C)Al2S3
D)AlSO4
E)Al2(SO4)3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
70
The correct formulas for the compounds in the figure are: 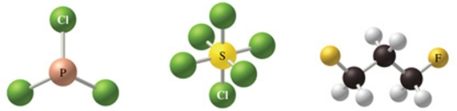
A)Cl3P, Cl6S, C3H2F6
B)PCl3, SCl6, C3H6F2
C)PCl3, SCl4, C3H6F3
D)P3Cl, S6Cl, C3H6F2
E)PCl3, SCl8, C3H6F2

A)Cl3P, Cl6S, C3H2F6
B)PCl3, SCl6, C3H6F2
C)PCl3, SCl4, C3H6F3
D)P3Cl, S6Cl, C3H6F2
E)PCl3, SCl8, C3H6F2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
71
The formula for copper(I) sulfide is:
A)CuS
B)CuS2
C)Cu2S
D)CuSO4
E)Cu2SO4
A)CuS
B)CuS2
C)Cu2S
D)CuSO4
E)Cu2SO4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following formula/name combinations is incorrect?
A)CS2 carbon disulfide
B)SO2 sulfur dioxide
C)N2O5 dinitrogen tetroxide
D)PF3 phosphorus trifluoride
E)P4O10 tetraphosphorus decoxide
A)CS2 carbon disulfide
B)SO2 sulfur dioxide
C)N2O5 dinitrogen tetroxide
D)PF3 phosphorus trifluoride
E)P4O10 tetraphosphorus decoxide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following formula/name combinations is incorrect?
A)N2O4 dinitrogen tetroxide
B)ClO2 dichlorine oxide
C)SO3 sulfur trioxide
D)CS2 carbon disulfide
E)PCl3 phosphorus trichloride
A)N2O4 dinitrogen tetroxide
B)ClO2 dichlorine oxide
C)SO3 sulfur trioxide
D)CS2 carbon disulfide
E)PCl3 phosphorus trichloride

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
74
The compound ClO3 is a dark brown solid powder that is not stable above 25 C.Name this molecular compound.
A)chlorine trioxide
B)chlorine oxide
C)chlorate
D)chlorite
E)chlorine(VI) oxide
A)chlorine trioxide
B)chlorine oxide
C)chlorate
D)chlorite
E)chlorine(VI) oxide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
75
Suppose you need iron(II) phosphate for an experiment.Which of these compounds would you use?
A)FeP
B)(Fe3P2 )
C)(Fe2(PO4)3 )
D)(Fe3(PO4)2 )
A)FeP
B)(Fe3P2 )
C)(Fe2(PO4)3 )
D)(Fe3(PO4)2 )

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
76
The correct formulas for the compounds in the figure are: 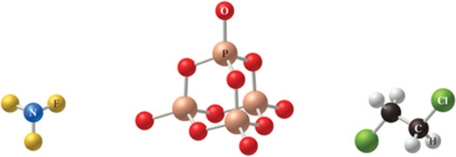
A)NF3, P4O10, C2H4Cl2
B)NF3, P4O8, C2H4Cl2
C)NF3, P4O10, C2H2Cl4
D)NF4, P3O10, C2H2Cl4
E)NF4, P3O10, C2H2Cl2

A)NF3, P4O10, C2H4Cl2
B)NF3, P4O8, C2H4Cl2
C)NF3, P4O10, C2H2Cl4
D)NF4, P3O10, C2H2Cl4
E)NF4, P3O10, C2H2Cl2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
77
The formula for calcium nitrate is:
A)CaNO3
B)Ca2NO3
C)Ca(NO3)2
D)Ca(NO3)3
E)Ca(NO2)2
A)CaNO3
B)Ca2NO3
C)Ca(NO3)2
D)Ca(NO3)3
E)Ca(NO2)2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
78
The formula for barium carbonate is:
A)Ba2CO3
B)BaCO3
C)BaCO2
D)BaCO
A)Ba2CO3
B)BaCO3
C)BaCO2
D)BaCO

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following compound names is not correct?
A)strontium dinitrate
B)sodium oxide
C)copper(II) carbonate
D)potassium permanganate
E)silver chloride
A)strontium dinitrate
B)sodium oxide
C)copper(II) carbonate
D)potassium permanganate
E)silver chloride

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is the correct name for the compound FeSO4?
A)iron(II) sulfate
B)iron(IV) sulfate
C)iron sulfate
D)iron sulfur tetroxide
E)iron sulfite
A)iron(II) sulfate
B)iron(IV) sulfate
C)iron sulfate
D)iron sulfur tetroxide
E)iron sulfite

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck


