Deck 6: Energy Flow in the Life of a Cell
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/21
Play
Full screen (f)
Deck 6: Energy Flow in the Life of a Cell
1
Define metabolism , and explain how reactions can be coupled to one another.
In this question, we are asked to define metabolism and explain the coupling of reactions to one another to achieve this idea.
All reactions tend towards lower energy states, even the reactions in living creatures. Thus, all chemistry and biochemistry tends to the "loss" of energy from systems, usually being released as heat.
This tendency to lose energy, contributing to the overall randomness of the universe, is known as entropy. Living things stand in contrast to entropy, as they are organized systems. To accomplish this organization, organisms take advantage of energy releasing reactions to power the reactions that require energy such as the building of proteins and DNA.
Living systems use energy-releasing reactions, called exergonic reactions, to drive energy absorbing reactions, called endergonic reactions. These endergonic reactions create complex molecules like proteins, and the energy they absorb is stored in the chemical bonds of those molecules. The sum of all exergonic and endergonic processes within an organism is known as metabolism.
All reactions tend towards lower energy states, even the reactions in living creatures. Thus, all chemistry and biochemistry tends to the "loss" of energy from systems, usually being released as heat.
This tendency to lose energy, contributing to the overall randomness of the universe, is known as entropy. Living things stand in contrast to entropy, as they are organized systems. To accomplish this organization, organisms take advantage of energy releasing reactions to power the reactions that require energy such as the building of proteins and DNA.
Living systems use energy-releasing reactions, called exergonic reactions, to drive energy absorbing reactions, called endergonic reactions. These endergonic reactions create complex molecules like proteins, and the energy they absorb is stored in the chemical bonds of those molecules. The sum of all exergonic and endergonic processes within an organism is known as metabolism.
2
The abbreviation ATP stands for____________. The molecule is synthesized by cells from__________ and___________. This synthesis requires an input of ____________, which is temporarily stored in ATP.
Energy is the requirement for all forms of life for their survival and it is generated in a living cell by various reactions by utilizing many metabolites.
ATP is a high energy molecule that gives energy to a cell in a very quick way. ATP stands for Adenosine Triphosphate and it is used as an energy fuel carrier in a living cell system.
The Adenosine Triphosphate is synthesized by cells from Adenosine Diphosphate and an inorganic phosphate molecule. This reaction of ATP synthesis is an endergonic reaction, and the absorbed energy is stored temporarily in ATP to fulfill further energy requirements in a cell.
So, the answer for first blank is Adenosine triphosphate , which is ATP, Adenosine diphosphate and phosphate is the answer for second and third blanks, which synthesizes ATP, and energy is the fourth answer, that is temporarily stored in ATP.
ATP is a high energy molecule that gives energy to a cell in a very quick way. ATP stands for Adenosine Triphosphate and it is used as an energy fuel carrier in a living cell system.
The Adenosine Triphosphate is synthesized by cells from Adenosine Diphosphate and an inorganic phosphate molecule. This reaction of ATP synthesis is an endergonic reaction, and the absorbed energy is stored temporarily in ATP to fulfill further energy requirements in a cell.
So, the answer for first blank is Adenosine triphosphate , which is ATP, Adenosine diphosphate and phosphate is the answer for second and third blanks, which synthesizes ATP, and energy is the fourth answer, that is temporarily stored in ATP.
3
Coupled reactions
A) are endergonic overall.
B) both synthesize and break down ATP.
C) are catalyzed by the same enzyme.
D) end with reactants that contain more energy than their products.
A) are endergonic overall.
B) both synthesize and break down ATP.
C) are catalyzed by the same enzyme.
D) end with reactants that contain more energy than their products.
The chemical reaction in which two reactions occur simultaneously, where the energy from one reaction is transferred to accelerate the other reaction it is termed as coupled reaction. Hence, both exergonic and endergonic reactions are coupled in this reaction.
In coupled reaction, the exergonic reaction provides excess amount of energy which is higher than the need to accelerate endergonic reaction. Thus, they are exergonic overall.
As the coupled reactions are exergonic, the high energy molecules are produced from low energy reactants. Thus, the reactants contain less energy compared to theit products.
The exergonic and endergonic reaction of a coupled reaction is catalyzed by same enzyme but, the coupled reaction is not catalyzed always by a same enzyme. The enzyme differs for a specific coupled reaction depending upon the reactants and products of the reaction.
Hence, options (a), (c), and (d) are incorrect, as none of them are a nature of coupled reaction.
In the coupled reactions the energy is released spontaneously during exergonic reaction and it is captured and synthesized as Adenosine Triphosphate (ATP) constantly. ATP synthesized is sequentially hydrolyzed to transfer energy for the coupled endergonic reaction.
Hence, the option (b) is correct.
In coupled reaction, the exergonic reaction provides excess amount of energy which is higher than the need to accelerate endergonic reaction. Thus, they are exergonic overall.
As the coupled reactions are exergonic, the high energy molecules are produced from low energy reactants. Thus, the reactants contain less energy compared to theit products.
The exergonic and endergonic reaction of a coupled reaction is catalyzed by same enzyme but, the coupled reaction is not catalyzed always by a same enzyme. The enzyme differs for a specific coupled reaction depending upon the reactants and products of the reaction.
Hence, options (a), (c), and (d) are incorrect, as none of them are a nature of coupled reaction.
In the coupled reactions the energy is released spontaneously during exergonic reaction and it is captured and synthesized as Adenosine Triphosphate (ATP) constantly. ATP synthesized is sequentially hydrolyzed to transfer energy for the coupled endergonic reaction.
Hence, the option (b) is correct.
4
What is activation energy? How do catalysts affect activation energy? How do catalysts affect the rate of reactions?

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
5
Enzymes are what type of biological molecule? _______________Enzymes promote reactions in cells by acting as biological___________ that lower the __________. Each enzyme possesses a region called a(n) _________that binds specific biological molecules.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
6
While vacuuming, you show off by telling a friend that you are using electrical energy to create a lower-entropy state. She replies that you are taking advantage of increasing solar entropy. Explain this conversation.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is False?
A) Allosteric inhibition is noncompetitive.
B) Allosteric regulation can either stimulate or inhibit enzyme activity.
C) Feedback inhibition is a form of allosteric regulation.
D) Competitive inhibition is a form of allosteric enzyme regulation.
A) Allosteric inhibition is noncompetitive.
B) Allosteric regulation can either stimulate or inhibit enzyme activity.
C) Feedback inhibition is a form of allosteric regulation.
D) Competitive inhibition is a form of allosteric enzyme regulation.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
8
According to the first law of thermodynamics, energy can be neither ______________ nor ______________. Energy occurs in two major forms: ______________, the energy of movement, and ______________, or stored energy.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
9
Compare breaking down glucose in a cell to setting it on fire with a match. What is the source of activation energy in each case?

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is True?
A) Enzymes increase activation energy requirements.
B) Activation energy is required to initiate exergonic reactions.
C) Heat cannot supply activation energy.
D) Stomach acid inactivates pepsin.
A) Enzymes increase activation energy requirements.
B) Activation energy is required to initiate exergonic reactions.
C) Heat cannot supply activation energy.
D) Stomach acid inactivates pepsin.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
11
Some poisons and drugs act by___________ enzymes. When a drug is similar to the enzyme's substrate, it acts as a(n) ____________inhibitor.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
12
Explain why organisms do not violate the second law of thermodynamics. What is the ultimate energy source for most forms of life on Earth?

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
13
Compare the mechanisms of competitive and noncompetitive inhibition of enzymes.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
14
Refute the following: "According to evolutionary theory, organisms have increased in complexity through time. However, an increase in complexity contradicts the second law of thermodynamics. Therefore, evolution is impossible."

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
15
Describe the structure and function of enzymes. How is enzyme activity regulated?

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
16
According to the second law of thermodynamics, when energy changes forms, some is always converted into ___________ useful forms. This tendency is called ___________________.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
17
Which is not an example of an exergonic reaction?
A) photosynthesis
B) a nuclear reaction in the sun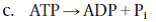
D) glucose breakdown
A) photosynthesis
B) a nuclear reaction in the sun
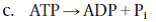
D) glucose breakdown

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
18
Define potential energy and kinetic energy and provide two specific examples of each. Explain how one form of energy can be converted into another. Will some energy be lost during this conversion? If so, what form will it take?

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
19
Can a bear use all the energy contained in the body of the fish it eats? Explain, and based on your explanation, predict and further explain whether a forest would likely have more predators or more prey animals (by weight)?

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
20
Once started, some reactions release energy and are called__________ reactions. Others require a net input of energy and are called ___________reactions. Which type of reaction will continue spontaneously once it starts?_____________. Which type of reaction allows the formation of complex biological molecules from simpler molecules?___________.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is True?
A) ATP is a long-term energy storage molecule.
B) ATP can carry energy from one cell to another.
C) ADP inhibits glucose breakdown in cells.
D) ATP can act as an allosteric regulator molecule.
A) ATP is a long-term energy storage molecule.
B) ATP can carry energy from one cell to another.
C) ADP inhibits glucose breakdown in cells.
D) ATP can act as an allosteric regulator molecule.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck


