Deck 2: The Chemical Basis of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/87
Play
Full screen (f)
Deck 2: The Chemical Basis of Life
1
Which of the groups participates exclusively in hydrophobic interactions? 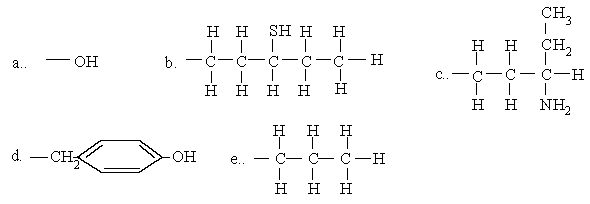
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e
e
2
Which interaction is most important in enhancing the solubility of macromolecules in water?
A)hydrophobic interactions
B)nonpolar covalent bonds
C)hydrogen bonds
D)van der Waals forces
E)Both hydrophobic interactions and nonpolar covalent bonds
A)hydrophobic interactions
B)nonpolar covalent bonds
C)hydrogen bonds
D)van der Waals forces
E)Both hydrophobic interactions and nonpolar covalent bonds
C
3
Which of the groups participates exclusively in hydrophilic interactions? 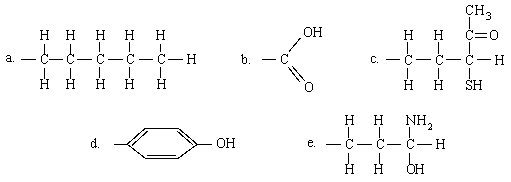
A)a
B)b
C)c
D)d
E)e

A)a
B)b
C)c
D)d
E)e
B
4
A highly focused treatment for brain cancer relies upon _____________________.
A)125I nuggets hemisperically arranged around the tumor, emitting convergent alpha particle beams which create the so-called alpha knife.
B)99Tc nuggets hemisperically arranged around the tumor, emitting convergent X ray beams which create the so-called X ray knife.
C)60Co nuggets hemisperically arranged around the tumor, emitting convergent gamma ray beams which create the so-called gamma knife.
D)99Mo nuggets hemisperically arranged around the tumor, emitting convergent beta ray beams which create the so-called beta knife.
A)125I nuggets hemisperically arranged around the tumor, emitting convergent alpha particle beams which create the so-called alpha knife.
B)99Tc nuggets hemisperically arranged around the tumor, emitting convergent X ray beams which create the so-called X ray knife.
C)60Co nuggets hemisperically arranged around the tumor, emitting convergent gamma ray beams which create the so-called gamma knife.
D)99Mo nuggets hemisperically arranged around the tumor, emitting convergent beta ray beams which create the so-called beta knife.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
5
The electronegativity of an atom is related to the:
A)number of electrons in its nucleus
B)number of neutrons in its nucleus
C)number of protons in its nucleus
D)the combined number of neutrons and protons in its nucleus
A)number of electrons in its nucleus
B)number of neutrons in its nucleus
C)number of protons in its nucleus
D)the combined number of neutrons and protons in its nucleus

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
6
Inert biological molecules like fats and waxes are chemically characterized by possessing many:
A)non-polar covalent bonds
B)ionic bonds
C)polar covalent bonds
D)oxygen, nitrogen or sulfur atoms
A)non-polar covalent bonds
B)ionic bonds
C)polar covalent bonds
D)oxygen, nitrogen or sulfur atoms

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
7
The isotope of hydrogen which is radioactive ________________________.
A)possesses three neutrons and is called tritium
B)possesses two neutrons and is called tritium
C)possesses three neutrons and is called deuterium
D)possesses two neutrons and is called deuterium
A)possesses three neutrons and is called tritium
B)possesses two neutrons and is called tritium
C)possesses three neutrons and is called deuterium
D)possesses two neutrons and is called deuterium

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
8
In a cell, where are strong ionic bonds most likely to be found?
A)in the cytoplasm
B)between DNA strands
C)deep in a protein's core where water is excluded
D)on the surface of a protein
E)on the surface of a lipid
A)in the cytoplasm
B)between DNA strands
C)deep in a protein's core where water is excluded
D)on the surface of a protein
E)on the surface of a lipid

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
9
Elements are likely to exhibit similar properties if:
A)they have the same number of electrons in their innermost orbital
B)they have the same number of electrons in their outermost orbital
C)they have the same number of electrons in their innermost electron shell
D)they have the same number of electrons in their outermost electron shell
A)they have the same number of electrons in their innermost orbital
B)they have the same number of electrons in their outermost orbital
C)they have the same number of electrons in their innermost electron shell
D)they have the same number of electrons in their outermost electron shell

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
10
Which of these atoms is the LEAST likely to form bonds with others?
A)neon
B)carbon
C)fluorine
D)lithium
E)hydrogen
A)neon
B)carbon
C)fluorine
D)lithium
E)hydrogen

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
11
The most stable atoms, and thus those that are typically nonreactive, are the atoms that have _______.
A)equal numbers of electrons and protons
B)equal numbers of electrons and neutrons
C)full inner electron shells
D)full outer electron shells
E)all covalent bonds
A)equal numbers of electrons and protons
B)equal numbers of electrons and neutrons
C)full inner electron shells
D)full outer electron shells
E)all covalent bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following elements are commonly found in biological molecules and strongly electronegative?
A)oxygen and carbon
B)oxygen and phosphorus
C)oxygen and nitrogen
D)carbon and nitrogen
E)carbon and sodium
A)oxygen and carbon
B)oxygen and phosphorus
C)oxygen and nitrogen
D)carbon and nitrogen
E)carbon and sodium

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
13
Which statement does NOT support the theory that aging may occur in response to cellular damage by free radicals?
A)Mice genetically engineered to express higher levels of catalase lived 20% longer than control mice.
B)Mice lacking mitochondrial superoxide dismutase died after a few weeks of life.
C)Bacteria and yeast cells incapable of expressing superoxide dismutase cannot live in an oxygen-containing environment.
D)Rats and mice fed high levels of antioxidants age at the same rate as those on a standard diet.
A)Mice genetically engineered to express higher levels of catalase lived 20% longer than control mice.
B)Mice lacking mitochondrial superoxide dismutase died after a few weeks of life.
C)Bacteria and yeast cells incapable of expressing superoxide dismutase cannot live in an oxygen-containing environment.
D)Rats and mice fed high levels of antioxidants age at the same rate as those on a standard diet.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
14
Which of these isotope emissions is LEAST likely to be useful for radiation therapy in cancer patients?
A)alpha particles
B)gamma rays
C)X-rays
D)beta particles
A)alpha particles
B)gamma rays
C)X-rays
D)beta particles

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
15
Under which circumstances would electrons be most likely to be shared equally?
A)when they are equidistant from nuclei
B)when they are equidistant from each other
C)when atoms of the same element are sharing them
D)when the atoms sharing them are different
A)when they are equidistant from nuclei
B)when they are equidistant from each other
C)when atoms of the same element are sharing them
D)when the atoms sharing them are different

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
16
The two elements most likely to exhibit similar reactive properties are:
A)lithium and neon
B)carbon and chlorine
C)oxygen and sulfur
D)sodium and fluorine
A)lithium and neon
B)carbon and chlorine
C)oxygen and sulfur
D)sodium and fluorine

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
17
Extremely high levels of electronegativity can result in the formation of:
A)non-polar covalent bonds
B)ionic bonds
C)polar covalent bonds
D)oxygen, nitrogen or sulfur atoms
A)non-polar covalent bonds
B)ionic bonds
C)polar covalent bonds
D)oxygen, nitrogen or sulfur atoms

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
18
What kind of noncovalent interaction is typified by interactions between two molecules that are so close together that they can experience weak attractive forces bonding them together?
A)hydrogen bond
B)ionic bond
C)hydrophobic interaction
D)polar covalent bond
E)van der Waals forces
A)hydrogen bond
B)ionic bond
C)hydrophobic interaction
D)polar covalent bond
E)van der Waals forces

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
19
Which of these bonds results from an unequal sharing of electrons?
A)ionic bond
B)polar covalent bond
C)triple bond
D)nonpolar covalent bond
A)ionic bond
B)polar covalent bond
C)triple bond
D)nonpolar covalent bond

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
20
Where are hydrophobic interactions most likely to occur?
A)on the surface of a water-soluble protein
B)the core of a water-soluble protein
C)in contact with water molecules
D)between two charged molecules
E)between two ions
A)on the surface of a water-soluble protein
B)the core of a water-soluble protein
C)in contact with water molecules
D)between two charged molecules
E)between two ions

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
21
If you want a solution which is basic to be neutralized by adding the smallest volume of an acid as possible, which of these choices would you use?
A)acetic acid
B)carbonic acid
C)hydrochloric acid
D)water
A)acetic acid
B)carbonic acid
C)hydrochloric acid
D)water

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
22
A pH shift from 4 to 7 creates a solution which is
A)100 times more acidic
B)1000 times more basic
C)3 times more acidic
D)300 times more basic
A)100 times more acidic
B)1000 times more basic
C)3 times more acidic
D)300 times more basic

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
23
The main buffer system operating to maintain stable blood pH is made up of:
A)Carbonic acid/bicarbonate ions
B)H2PO4-/HPO42-
C)acetate/acetic acid
D)chloride ions/ HCl
A)Carbonic acid/bicarbonate ions
B)H2PO4-/HPO42-
C)acetate/acetic acid
D)chloride ions/ HCl

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
24
Organic molecules are currently defined by which characteristic?
A)derived from a living organism
B)based on C-C bonds
C)possessing C-O bonds
D)containing C-H bonds
A)derived from a living organism
B)based on C-C bonds
C)possessing C-O bonds
D)containing C-H bonds

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
25
A buffer has all of the following characteristics EXCEPT:
A)the ability to protect cells from fluctuations in pH
B)the ability to protect cells from fluctuations in temperature
C)able to react with hydrogen ions
D)able to react with hydroxyl ions
A)the ability to protect cells from fluctuations in pH
B)the ability to protect cells from fluctuations in temperature
C)able to react with hydrogen ions
D)able to react with hydroxyl ions

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
26
Why is silicon not suitable for making covalent bonds stable and strong enough to form the basis of living organisms, even though it is just below carbon on the periodic table?
A)Silicon is too large for its nucleus to attract the valence electrons of neighboring atoms in order to form covalent bonds.
B)Silicon is too small for its nucleus to attract the valence electrons of neighboring atoms in order to form covalent bonds.
C)Silicon is too large for its nucleus to attract the protons of neighboring atoms in order to form covalent bonds.
D)Silicon is too small for its nucleus to attract the protons of neighboring atoms in order to form covalent bonds.
A)Silicon is too large for its nucleus to attract the valence electrons of neighboring atoms in order to form covalent bonds.
B)Silicon is too small for its nucleus to attract the valence electrons of neighboring atoms in order to form covalent bonds.
C)Silicon is too large for its nucleus to attract the protons of neighboring atoms in order to form covalent bonds.
D)Silicon is too small for its nucleus to attract the protons of neighboring atoms in order to form covalent bonds.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
27
The historic meaning of an organic molecule was that the molecule was derived from a living organism and contained carbon. Today we call such molecules __________________.
A)inorganics
B)polymers
C)biochemicals
D)cyclic
A)inorganics
B)polymers
C)biochemicals
D)cyclic

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
28
A release of hydrogen ions to a solution would most likely ____________.
A)raise pH
B)lower pH
C)buffer pH
D)change salinity
E)not affect pH
A)raise pH
B)lower pH
C)buffer pH
D)change salinity
E)not affect pH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
29
Chemists describe ionic bonds as strong bonds while cell biologists consider them relatively weak bonds. This can be explained due to the fact that:
A)chemists are usually considering free ionic bonds between charged atoms.
B)cell biologists are usually comparing ionic bonds to much stronger hydrogen bonds.
C)chemists examine ionic bonds only in the context of an aqueous cellular environment.
D)cell biologists overlook the role of water in reducing the strength of ionic bonds.
A)chemists are usually considering free ionic bonds between charged atoms.
B)cell biologists are usually comparing ionic bonds to much stronger hydrogen bonds.
C)chemists examine ionic bonds only in the context of an aqueous cellular environment.
D)cell biologists overlook the role of water in reducing the strength of ionic bonds.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
30
Water's life-supporting properties include all of the following EXCEPT:
A)high boiling point compatible with temperate extremes of summer allowing energy to be released through sweat
B)protection from cold and damaging radiation by providing the matrix around which the cellular framework is constructed.
C)high probability of bonding with nonpolar molecules
D)ability to dissociate into OH- and H+ units within the cell
A)high boiling point compatible with temperate extremes of summer allowing energy to be released through sweat
B)protection from cold and damaging radiation by providing the matrix around which the cellular framework is constructed.
C)high probability of bonding with nonpolar molecules
D)ability to dissociate into OH- and H+ units within the cell

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
31
The optimal interatomic distance for strong Van der Waals interactions between atoms of two molecules is about:
A)1 angstrom
B)2 angstroms
C)4 angstroms
D)greater than 8 angstroms
A)1 angstrom
B)2 angstroms
C)4 angstroms
D)greater than 8 angstroms

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
32
The low-molecular-weight building blocks of polymers are called _______.
A)minipolymers
B)monoblocks
C)monomers
D)portions
E)octamers
A)minipolymers
B)monoblocks
C)monomers
D)portions
E)octamers

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
33
A molecule that is capable of releasing or donating a hydrogen ion is termed _______.
A)a base
B)a hydrion
C)an acid
D)an anachronism
E)an amphipath
A)a base
B)a hydrion
C)an acid
D)an anachronism
E)an amphipath

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
34
Van der Waals forces are the result of:
A)ionic attractions
B)hydrophilic molecular attractions to one another
C)aggregation to exclude water from hydrophobic molecular faces
D)transient asymmetric electron density shifts
A)ionic attractions
B)hydrophilic molecular attractions to one another
C)aggregation to exclude water from hydrophobic molecular faces
D)transient asymmetric electron density shifts

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
35
A pH shift from 10 to 8 creates a solution which is
A)100 times more acidic
B)1000 times more basic
C)2 times more acidic
D)200 times more basic
A)100 times more acidic
B)1000 times more basic
C)2 times more acidic
D)200 times more basic

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is NOT a macromolecule formed by polymerization?
A)proteins
B)lipids
C)polynucleotides
D)polysaccharides
E)DNA
A)proteins
B)lipids
C)polynucleotides
D)polysaccharides
E)DNA

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
37
At higher proton concentrations, proteins are likely to possess:
A)more NH2 groups and more NH3+ groups
B)fewer NH2 groups and fewer NH3+ groups
C)more NH2 groups and fewer NH3+ groups
D)fewer NH2 groups and more NH3+ groups
A)more NH2 groups and more NH3+ groups
B)fewer NH2 groups and fewer NH3+ groups
C)more NH2 groups and fewer NH3+ groups
D)fewer NH2 groups and more NH3+ groups

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
38
Hyperventilation can create the potential for blood pH to rise to a harmful level. Why are hyperventilating individuals suffering from panic attacks advised to breathe in and out from within a paper bag?
A)the bag increases the air pressure, allowing more hydrogen ions to dissolve in the blood
B)the additional CO2 combines with water to produce carbonic acid, which, in turn dissociates to bicarbonate and protons, thereby lowering pH
C)the additional CO2 combines with water to produce bicarbonate, which, in turn dissociates to carbonic acid and protons, thereby lowering pH
D)the additional CO2 combines with water to produce carbonic acid, which, in turn dissociates to bicarbonate and protons, thereby raising pH
A)the bag increases the air pressure, allowing more hydrogen ions to dissolve in the blood
B)the additional CO2 combines with water to produce carbonic acid, which, in turn dissociates to bicarbonate and protons, thereby lowering pH
C)the additional CO2 combines with water to produce bicarbonate, which, in turn dissociates to carbonic acid and protons, thereby lowering pH
D)the additional CO2 combines with water to produce carbonic acid, which, in turn dissociates to bicarbonate and protons, thereby raising pH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
39
Reactions in which a proton may participate include:
A)formation of an amine group by combining with a sugar hydroxyl group
B)formation of an amine group by combining with a hydronium ion
C)formation of water by combining with a sugar hydroxyl group
D)formation of water by combining with a free hydroxyl group
A)formation of an amine group by combining with a sugar hydroxyl group
B)formation of an amine group by combining with a hydronium ion
C)formation of water by combining with a sugar hydroxyl group
D)formation of water by combining with a free hydroxyl group

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following tripeptides would be most likely to be soluble in an organic (hydrophobic)solvent like benzene?
A)N - phenylalanine - alanine - glycine - C
B)N - leucine - alanine - lysine - C
C)N - proline - phenylalanine - leucine - C
D)N - arginine - lysine - proline - C
E)N - glutamate - aspartate - glycine - C
A)N - phenylalanine - alanine - glycine - C
B)N - leucine - alanine - lysine - C
C)N - proline - phenylalanine - leucine - C
D)N - arginine - lysine - proline - C
E)N - glutamate - aspartate - glycine - C

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
41
How do amino acids like hydroxylysine and thyroxine, which are not among the 20 amino acids inserted into amino acid chains, get into proteins?
A)They are inserted due to mutations.
B)They are the result of the alteration of R groups of the 20 amino acids after their incorporation into the polypeptide.
C)They occur as carboxyl groups are altered, reversing the chirality of the molecules.
D)Insertion of the amino acid into the polypeptide causes bonds to break and new amino acids to form.
A)They are inserted due to mutations.
B)They are the result of the alteration of R groups of the 20 amino acids after their incorporation into the polypeptide.
C)They occur as carboxyl groups are altered, reversing the chirality of the molecules.
D)Insertion of the amino acid into the polypeptide causes bonds to break and new amino acids to form.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
42
An informative test for the diagnosis of diabetes is to monitor levels of Hemoglobin A1c, produced through the reaction of hemoglobin and __________________.
A)the linear aldose form of glucose
B)the cyclic isoform of glucose
C)the chair form of glucose
D)the Haworth projection form of glucose
A)the linear aldose form of glucose
B)the cyclic isoform of glucose
C)the chair form of glucose
D)the Haworth projection form of glucose

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
43
Which group of plants have nitrogen fixing symbionts?
A)monocots
B)dicots
C)legumes
D)rhizobia
E)bacteroids
A)monocots
B)dicots
C)legumes
D)rhizobia
E)bacteroids

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is a nucleotide?
A)phosphate + ribose + deoxyribose
B)adenine + deoxyribose
C)sugar + nitrogenous base
D)adenine + ribose + phosphate
A)phosphate + ribose + deoxyribose
B)adenine + deoxyribose
C)sugar + nitrogenous base
D)adenine + ribose + phosphate

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
45
Why do sugars tend to be highly water soluble?
A)they have only a few hydroxyl groups
B)they have large numbers of hydroxyl groups
C)they have large numbers of sulfhydryl groups
D)they have large numbers of methyl groups
E)they have small molecular weights
A)they have only a few hydroxyl groups
B)they have large numbers of hydroxyl groups
C)they have large numbers of sulfhydryl groups
D)they have large numbers of methyl groups
E)they have small molecular weights

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
46
Which organism would be a good candidate for researching genes related to nitrogen fixation?
A)rhizobia
B)algae
C)leguminous plants
D)non-leguminous plants
A)rhizobia
B)algae
C)leguminous plants
D)non-leguminous plants

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
47
Carbon's suitability as the molecular framework for biochemicals stems from its:
A)large nucleus containing a high ratio of neutrons
B)almost full outer shell of electrons, allowing for the formation of linear, branched and cyclic molecules
C)ability to form elongated polymers
D)high electronegativity
A)large nucleus containing a high ratio of neutrons
B)almost full outer shell of electrons, allowing for the formation of linear, branched and cyclic molecules
C)ability to form elongated polymers
D)high electronegativity

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
48
What level of structure in proteins is held together by R group interactions between different polypeptides?
A)primary structure
B)secondary structure
C)tertiary structure
D)quaternary structure
A)primary structure
B)secondary structure
C)tertiary structure
D)quaternary structure

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
49
Proteins are often composed of two or more distinct modules that fold up independently of one another. They often represent parts of a protein that function in a semi-independent manner. These modules are called ______.
A)protein motifs
B)functionals
C)domains
D)dominoes
A)protein motifs
B)functionals
C)domains
D)dominoes

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
50
What type of protein secondary structure is characterized as being highly extensible because of its coiled structure?
A) -pleated sheet
B)proline kink
C) -helix
D)globular
A) -pleated sheet
B)proline kink
C) -helix
D)globular

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
51
Which element can only be obtained by plants through mineral uptake, rather than via atmospheric fixation by the plant or its symbiont?
A)phosphorus
B)carbon
C)nitrogen
D)oxygen
A)phosphorus
B)carbon
C)nitrogen
D)oxygen

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
52
Which polysaccharide bond cannot be broken by mammalian enzymes that normally digest polysaccharides?
A) (1->4)glycosidic linkages
B) (1->4)glycosidic linkages
C) (1->6)glycosidic linkages
D) (1->6)glycosidic linkages
E)phosphate ester linkages
A) (1->4)glycosidic linkages
B) (1->4)glycosidic linkages
C) (1->6)glycosidic linkages
D) (1->6)glycosidic linkages
E)phosphate ester linkages

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
53
Select the order in which you might expect molecules involved in the synthesis of a polymer to be present as a cell grows.
A)precursor, metabolic intermediate, miscellaneous molecule
B)metabolic intermediate, miscellaneous molecule, macromolecule
C)precursor, metabolic intermediate, macromolecule
D)macromolecule, precursor, miscelleaneous molecule
A)precursor, metabolic intermediate, miscellaneous molecule
B)metabolic intermediate, miscellaneous molecule, macromolecule
C)precursor, metabolic intermediate, macromolecule
D)macromolecule, precursor, miscelleaneous molecule

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
54
What bond is responsible for the branch points in glycogen and amylopectin?
A) (1->4)glycosidic linkages
B) (1->4)glycosidic linkages
C) (1->6)glycosidic linkages
D) (1->6)glycosidic linkages
E)3'-5' phosphodiester linkages
A) (1->4)glycosidic linkages
B) (1->4)glycosidic linkages
C) (1->6)glycosidic linkages
D) (1->6)glycosidic linkages
E)3'-5' phosphodiester linkages

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
55
The -pleated sheet is characterized by the orientation of ______ to the molecular axis.
A)H bonds parallel
B)H bonds perpendicular
C)ionic bonds parallel
D)ionic bonds perpendicular
E)peptide bonds perpendicular
A)H bonds parallel
B)H bonds perpendicular
C)ionic bonds parallel
D)ionic bonds perpendicular
E)peptide bonds perpendicular

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
56
The endosymbiotic theory proposes that bacteria were engulfed by ancestral eukaryotic cells and sheltered within their cytoplasm whilst providing a survival advantage to the eukaryotic cell as well. Mitochondria and chloroplasts, now organelles, possess DNA with sequence homology to rickettsia and cyanobacteria, respectively. Which of the choices below best describes an organelle-like structure with a similar beneficial relationship when considering plant growth in nitrogen-limited soils?
A)rhizobia
B)bacteroids
C)legumes
D)cyanobacteria
E)all are possible correct choices
A)rhizobia
B)bacteroids
C)legumes
D)cyanobacteria
E)all are possible correct choices

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
57
If a polypeptide is 100 amino acids long, and could be made of any of the 20 amino acids in any order, what is the total number of possible variations for this polypetide?
A)10020
B)2,000
C)20100
D)20101
E)20
A)10020
B)2,000
C)20100
D)20101
E)20

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
58
Which of these amino acids is most likely to be found in the core of a protein?
A)methionine
B)asparagine
C)serine
D)threonine
E)glutamic acid
A)methionine
B)asparagine
C)serine
D)threonine
E)glutamic acid

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
59
Which of these statements regarding glyceraldehyde is NOT correct?
A)glyceraldehyde stereoisomers exhibit optical activity by rotating plane-polarized light in opposite directions
B)glyceraldehyde stereoisomers cannot be superimposed on one another
C)glyceraldehyde is one of several three-carbon aldoses
D)glyceraldehyde's second carbon is covalently bound to H, OH, CHO and COOH groups
A)glyceraldehyde stereoisomers exhibit optical activity by rotating plane-polarized light in opposite directions
B)glyceraldehyde stereoisomers cannot be superimposed on one another
C)glyceraldehyde is one of several three-carbon aldoses
D)glyceraldehyde's second carbon is covalently bound to H, OH, CHO and COOH groups

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
60
Which of these functional groups is the LEAST polar? Refer to Table 2.2 for more information if you are uncertain of the molecular structure of each one.
A)CH3
B)OH
C)COOH
D)SH
A)CH3
B)OH
C)COOH
D)SH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
61
Many so-called temperature-sensitive mutations have been discovered in a wide variety of organisms. These are proteins that are non-functional at higher temperatures, while, at lower temperatures (often just a few degrees lower), they function normally. For example, the coloration patterns in Siamese Cats arise from a temperature-sensitive mutation. An enzyme required for the synthesis of dark pigment is unable to function in areas close to the body where normal physiological temperatures prevail. However, at the tips of the ears, paws, the tip of the tail and other extremities where the temperature is slightly lower, the enzyme works correctly and dark pigment is produced. What is happening at the molecular level that explains this?
A)the enzyme responsible for pigment production is more sensitive to cold than others in the animal cells and unfolds in the cooler environment of the extremities
B)the enzyme responsible for pigment production is more sensitive to heat than others in the animal cells and unfoldsin the cooler environment of the extremities
C)the enzyme responsible for pigment production is more sensitive to cold than others in the animal cells and maintains activity in the cooler environment of the extremities
D)the enzyme responsible for pigment production is more sensitive to heat than others in the animal cells and maintains activity in the cooler environment of the extremities
A)the enzyme responsible for pigment production is more sensitive to cold than others in the animal cells and unfolds in the cooler environment of the extremities
B)the enzyme responsible for pigment production is more sensitive to heat than others in the animal cells and unfoldsin the cooler environment of the extremities
C)the enzyme responsible for pigment production is more sensitive to cold than others in the animal cells and maintains activity in the cooler environment of the extremities
D)the enzyme responsible for pigment production is more sensitive to heat than others in the animal cells and maintains activity in the cooler environment of the extremities

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
62
Not all proteins are able to renature. When exposed to heat or some other denaturing treatment , some proteins are irreversibly denatured, while others can renature when the denaturant is removed. What is an example of a protein demonstrated to refold correctly?
A)hemoglobin
B)urease
C)egg yolk protein
D)ribonuclease A
A)hemoglobin
B)urease
C)egg yolk protein
D)ribonuclease A

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
63
The nucleic acid precursor comprising one nitrogenous base and one sugar is known as a:
A)nucleoside
B)nucleotide
C)pyrimidine
D)purine
A)nucleoside
B)nucleotide
C)pyrimidine
D)purine

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
64
Examine the structures of cholesterol, testosterone and estrogen shown in Figure 2.23 (Or provide the figure for the Q). If we know that cholesterol is the precursor for synthesizing the other sex hormones, which of the statements below is likely to be CORRECT?
A)cholesterol will require demethylation to be converted into testosterone but not to be converted into estrogen
B)cholesterol will require demethylation to be converted into both testosterone and estrogen
C)cholesterol will require demethylation to be converted into estrogen but not to be converted into testosterone
D)all statements are incorrect
A)cholesterol will require demethylation to be converted into testosterone but not to be converted into estrogen
B)cholesterol will require demethylation to be converted into both testosterone and estrogen
C)cholesterol will require demethylation to be converted into estrogen but not to be converted into testosterone
D)all statements are incorrect

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
65
You are studying a protein. It binds to elongating polypeptide chains as they emerge from an exit channel within the ribosome's large subunit. It appears to prevent partially formed or nascent polypeptides from binding to other proteins in the cytosol, which might cause them either to aggregate or misfold. What kind of proteins is this likely to be?
A)Hsp70 chaperone
B)TRiC chaperonin
C)heat shock protein
D)ribosomal protein
A)Hsp70 chaperone
B)TRiC chaperonin
C)heat shock protein
D)ribosomal protein

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
66
You discover a novel protozoan which seems able to exist in the extreme salinity of the Dead Sea. Comparative examination of its enzyme structures against those of non-halophilic protozoa are most likely to reveal that ________________.
A)the novel protozoan possesses enzymes where the outer protein surface has many acidic amino acid residues
B)the novel protozoan possesses enzymes where the outer protein surface has many nonpolar amino acid residues
C)the novel protozoan possesses enzymes where the outer protein surface has many cysteine-associated disulfide bridges
D)all are equally likely possibilities
A)the novel protozoan possesses enzymes where the outer protein surface has many acidic amino acid residues
B)the novel protozoan possesses enzymes where the outer protein surface has many nonpolar amino acid residues
C)the novel protozoan possesses enzymes where the outer protein surface has many cysteine-associated disulfide bridges
D)all are equally likely possibilities

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
67
If an organism lives in an environment where significant temperature fluctuations occur, and is capable of altering the fatty acid composition of its phospholipid bilayer, which of these choices would best allow membrane integrity to remain stable? (Select all that apply)
A)increasing stearic acid incorporation during cold weather
B)decreasing stearic acid incorporation during cold weather
C)increasing stearic acid incorporation during hot weather
D)decreasing stearic acid incorporation during hot weather
A)increasing stearic acid incorporation during cold weather
B)decreasing stearic acid incorporation during cold weather
C)increasing stearic acid incorporation during hot weather
D)decreasing stearic acid incorporation during hot weather

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
68
You are working with an enzyme altase that you denature in the presence of urea. If altase were denatured no further by the addition of mercaptoethanol, what would that suggest to you about the enzyme?
A)The enzyme probably contained no positively charged amino acids since these are neutralized by mercaptoethanol.
B)The enzyme probably contained no acidic amino acids since these are neutralized by mercaptoethanol.
C)The enzyme probably contained no disulfide linkages since mercaptoethanol breaks such linkages.
D)Mercaptoethanol and urea denature enzymes in the same manner, so the observation lacks significance.
A)The enzyme probably contained no positively charged amino acids since these are neutralized by mercaptoethanol.
B)The enzyme probably contained no acidic amino acids since these are neutralized by mercaptoethanol.
C)The enzyme probably contained no disulfide linkages since mercaptoethanol breaks such linkages.
D)Mercaptoethanol and urea denature enzymes in the same manner, so the observation lacks significance.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
69
Which lipid is most likely to remain solid at room temperature?
A)tristearate
B)vegetable oil
C)polyunsaturated fat
D)linseed oil
A)tristearate
B)vegetable oil
C)polyunsaturated fat
D)linseed oil

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
70
Which of these functional groups would make a biochemical more soluble in water? (Select all that apply)
A)CH3
B)OH
C)COOH
D)SH
A)CH3
B)OH
C)COOH
D)SH

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
71
Select the correct statements regarding carbohydrate structure: (Select all that apply)
A)If the carbonyl group of a sugar has an internal placing, the sugar is termed a ketose, such as glucose.
B)If the carbonyl group of a sugar has an internal placing, the sugar is termed a ketose, such as fructose.
C)If the carbonyl group of a sugar has a terminal placing, the sugar is termed an aldose, such as glucose.
D)If the carbonyl group of a sugar has a terminal placing, the sugar is termed an aldose, such as fructose.
A)If the carbonyl group of a sugar has an internal placing, the sugar is termed a ketose, such as glucose.
B)If the carbonyl group of a sugar has an internal placing, the sugar is termed a ketose, such as fructose.
C)If the carbonyl group of a sugar has a terminal placing, the sugar is termed an aldose, such as glucose.
D)If the carbonyl group of a sugar has a terminal placing, the sugar is termed an aldose, such as fructose.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
72
Select the durable structural polysaccharides from the choices provided. (Select all that apply)
A)glucose
B)starch
C)cellulose
D)chitin
E)glycosaminoglycan
A)glucose
B)starch
C)cellulose
D)chitin
E)glycosaminoglycan

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
73
Biological roles for carbohydrates include: (Select all that apply)
A)structural molecules
B)catalytic molecules
C)energy storage molecules
D)repositories of genetic information
A)structural molecules
B)catalytic molecules
C)energy storage molecules
D)repositories of genetic information

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
74
The process by which proteins and RNA form in an elastic conformation is known as:
A)aqueous phase separation
B)gelation
C)intrinsically disordered domain formation
D)intrinsically organized domain formation
A)aqueous phase separation
B)gelation
C)intrinsically disordered domain formation
D)intrinsically organized domain formation

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
75
Fatty acids with a polar carboxyl group and a nonpolar hydrocarbon chain are considered ___________________ molecules.
A)entirely hydrophobic
B)entirely hydrophilic
C)amphipathic
D)non-reactive
A)entirely hydrophobic
B)entirely hydrophilic
C)amphipathic
D)non-reactive

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following experimental assemblies provides the LEAST compelling evidence that life processes are often self-directed?
A)The 1955 observation that tobacco mosaic virus spontaneously assembles.
B)Nomura's 1960's experiment demonstrated spontaneous assembly of a bacterial 30S ribosomal unit from 21 purified proteins and ribosomal RNA.
C)Removal of protein S16 from the bacterial 30S ribosomal protein/RNA mix in Nomura's experiment prevented self-assembly, providing evidence that the assembly is self-directed and sequential steps are essential.
D)Bacterial ribosome assembly is achieved inside cells in as little as 10 minutes, while in vitro 2 hours at elevated temperatures is required.
A)The 1955 observation that tobacco mosaic virus spontaneously assembles.
B)Nomura's 1960's experiment demonstrated spontaneous assembly of a bacterial 30S ribosomal unit from 21 purified proteins and ribosomal RNA.
C)Removal of protein S16 from the bacterial 30S ribosomal protein/RNA mix in Nomura's experiment prevented self-assembly, providing evidence that the assembly is self-directed and sequential steps are essential.
D)Bacterial ribosome assembly is achieved inside cells in as little as 10 minutes, while in vitro 2 hours at elevated temperatures is required.

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
77
A single-ringed nitrogenous base found in nucleic acid precursors is termed a ___________________.
A)nucleoside
B)nucleotide
C)pyrimidine
D)purine
A)nucleoside
B)nucleotide
C)pyrimidine
D)purine

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
78
In a phospholipid, the end of the molecule likely to face towards a nonpolar environment is ________________.
A)the end with the choline group
B)the end with the phosphate group
C)the region with the glycerol backbone
D)the end with the fatty acid chains
A)the end with the choline group
B)the end with the phosphate group
C)the region with the glycerol backbone
D)the end with the fatty acid chains

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
79
TAP-tag mass spectrometry is a technique employed in the field of science best described as:
A)genomics
B)proteomics
C)interactomics
D)lipidomics
A)genomics
B)proteomics
C)interactomics
D)lipidomics

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck
80
It is thought that most human diseases leave telltale patterns among the thousands of proteins present in the blood or other bodily fluids. It was hoped that analysis of the proteins present in the blood would help in the diagnosis of human disease; however, thus far, searches for these proteins in blood or bodily fluids have been largely unsuccessful and their use in diagnostics largely unreliable. What are these telltale patterns of proteins called?
A)chaperonins
B)biomarkers
C)proteomes
D)peptide mass fingerprints
A)chaperonins
B)biomarkers
C)proteomes
D)peptide mass fingerprints

Unlock Deck
Unlock for access to all 87 flashcards in this deck.
Unlock Deck
k this deck


