Deck 13: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/137
Play
Full screen (f)
Deck 13: Acids and Bases
1
The substance NaOH(aq)is
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.
a strong base.
2
Which of the following statements regarding acids is incorrect?
A)An acid will react with an active metal.
B)An acid is a substance that will change the color of blue litmus paper.
C)An acid is a substance that would taste sour.
D)An acid is a substance that produces hydrogen ions, H+, (or H3O+)in aqueous solution.
E)An acid is always a strong electrolyte.
A)An acid will react with an active metal.
B)An acid is a substance that will change the color of blue litmus paper.
C)An acid is a substance that would taste sour.
D)An acid is a substance that produces hydrogen ions, H+, (or H3O+)in aqueous solution.
E)An acid is always a strong electrolyte.
An acid is always a strong electrolyte.
3
Which of the following statements regarding bases is incorrect?
A)A base is a substance that may act as an antacid.
B)A base is a substance that will not change the color of red litmus paper.
C)A base is a substance that tastes bitter.
D)A base is a substance that produces hydroxide ions, OH−, in aqueous solution.
E)A base is a substance that is an electrolyte.
A)A base is a substance that may act as an antacid.
B)A base is a substance that will not change the color of red litmus paper.
C)A base is a substance that tastes bitter.
D)A base is a substance that produces hydroxide ions, OH−, in aqueous solution.
E)A base is a substance that is an electrolyte.
A base is a substance that will not change the color of red litmus paper.
4
Select the two Brønsted-Lowry acids in the following equation: HF(aq)+ H2O(l)⇌ F−(aq)+ H3O+(aq)
A)HF and H2O
B)HF and F−
C)H2O and F−
D)HF and H3O+
E)H3O+ and F−
A)HF and H2O
B)HF and F−
C)H2O and F−
D)HF and H3O+
E)H3O+ and F−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
5
The substance HNO3(aq)is
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
6
List the acid, base, conjugate acid, and conjugate base, in that order, for the following reaction: HOCl(aq)+ H2O(l)⇌ OCl−(aq)+ H3O+(aq)
A)HOCl, H2O, OCl−, H3O+
B)H2O, HOCl, OCl−, H3O+
C)HOCl, H2O, H3O+, OCl−
D)H2O, HOCl, H3O+, OCl−
E)OCl−, H3O+, H2O, HOCl
A)HOCl, H2O, OCl−, H3O+
B)H2O, HOCl, OCl−, H3O+
C)HOCl, H2O, H3O+, OCl−
D)H2O, HOCl, H3O+, OCl−
E)OCl−, H3O+, H2O, HOCl

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
7
Select the two Brønsted-Lowry acids in the following equation: HNO2(aq)+ H2O(l)⇌ NO2−(aq)+ H3O+(aq)
A)HNO2 and H2O
B)HNO2 and NO2−
C)HNO2 and H3O+
D)H2O and H3O+
E)NO2− and H3O+
A)HNO2 and H2O
B)HNO2 and NO2−
C)HNO2 and H3O+
D)H2O and H3O+
E)NO2− and H3O+

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following bases is not commonly found in household products or antacids?
A)sodium hydroxide, NaOH
B)ammonia, NH3
C)calcium carbonate, CaCO3
D)magnesium hydroxide, Mg(OH)2
E)methyl amine, CH3NH2
A)sodium hydroxide, NaOH
B)ammonia, NH3
C)calcium carbonate, CaCO3
D)magnesium hydroxide, Mg(OH)2
E)methyl amine, CH3NH2

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
9
List the acid, base, conjugate acid, and conjugate base, in that order, for the following reaction: CH3CO2H(aq)+ H2O(l)⇌ CH3CO2−(aq)+ H3O+(aq)
A)CH3CO2H, H2O, CH3CO2−, H3O+
B)H2O, CH3CO2H, CH3CO2−, H3O+
C)CH3CO2H, H2O, H3O+, CH3CO2−
D)H2O, CH3CO2H, H3O+, CH3CO2−
E)CH3CO2−, H3O+, H2O, CH3CO2H
A)CH3CO2H, H2O, CH3CO2−, H3O+
B)H2O, CH3CO2H, CH3CO2−, H3O+
C)CH3CO2H, H2O, H3O+, CH3CO2−
D)H2O, CH3CO2H, H3O+, CH3CO2−
E)CH3CO2−, H3O+, H2O, CH3CO2H

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
10
The substance NH3(aq)is
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
11
When the following reaction goes in the reverse direction (from products to reactants), what is the acid? HF(aq)+ H2O(l)⇌ F− (aq)+ H3O+(aq)
A)HF
B)H2O
C)F−
D)H3O
E)both F− and H3O
A)HF
B)H2O
C)F−
D)H3O
E)both F− and H3O

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is not an attribute of an acid?
A)It provides H+ ions in aqueous solution.
B)It is a nonelectrolyte.
C)It tastes sour.
D)It reacts with some organic dyes to cause them to change color.
E)It reacts with active metals.
A)It provides H+ ions in aqueous solution.
B)It is a nonelectrolyte.
C)It tastes sour.
D)It reacts with some organic dyes to cause them to change color.
E)It reacts with active metals.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following acids is not normally found in foods or beverages?
A)citric acid
B)acetic acid
C)phosphoric acid
D)carbonic acid
E)sulfuric acid
A)citric acid
B)acetic acid
C)phosphoric acid
D)carbonic acid
E)sulfuric acid

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is not an attribute of a base?
A)It provides OH− ions in aqueous solution.
B)It is an electrolyte.
C)It reacts with some organic dyes to cause them to change color.
D)It has the ability to neutralize acids.
E)It would taste sour.
A)It provides OH− ions in aqueous solution.
B)It is an electrolyte.
C)It reacts with some organic dyes to cause them to change color.
D)It has the ability to neutralize acids.
E)It would taste sour.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
15
When the following reaction goes in the reverse direction (from products to reactants), what is the base? HCN(aq)+ H2O(l)⇌ CN− (aq)+ H3O+(aq)
A)HCN
B)H2O
C)CN−
D)H3O+
E)both CN− and H3O+
A)HCN
B)H2O
C)CN−
D)H3O+
E)both CN− and H3O+

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
16
When the following reaction goes in the reverse direction (from products to reactants), what is the acid? HCN(aq)+ H2O(l)⇌ CN− (aq)+ H3O+(aq)
A)HCN
B)H2O
C)CN−
D)H3O+
E)both CN− and H3O+
A)HCN
B)H2O
C)CN−
D)H3O+
E)both CN− and H3O+

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
17
When the following reaction goes in the reverse direction (from products to reactants), what is the base? HF(aq)+ H2O(l)⇌ F−(aq)+ H3O+(aq)
A)HF
B)H2O
C)F−
D)H3O+
E)both F− and H3O+
A)HF
B)H2O
C)F−
D)H3O+
E)both F− and H3O+

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
18
The substance Mg(OH)2(aq)is
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
19
The substance HCl(aq)is
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.
A)a strong acid.
B)a weak acid.
C)a strong base.
D)a weak base.
E)neither an acid nor a base.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
20
Select the two Brønsted-Lowry acids in the following equation: HCN(aq)+ H2O(l)⇌ CN−(aq)+ H3O+(aq)
A)HCN and H2O
B)HCN and CN−
C)H2O and CN−
D)HCN and H3O+
E)H3O+ and CN−
A)HCN and H2O
B)HCN and CN−
C)H2O and CN−
D)HCN and H3O+
E)H3O+ and CN−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
21
The conjugate base of HCO3- is:
A)H2CO3
B)HCO2−
C)CO32−
D)CO2
E)H3CO3+
A)H2CO3
B)HCO2−
C)CO32−
D)CO2
E)H3CO3+

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
22
When acetate ion, CH3CO2−, reacts with water, what are the products?
A)H+(aq)and OH−(aq)
B)CH3CO2+(aq)and OH−(aq)
C)CH3CO2+(aq)and H2O(aq)
D)CH3CO2+(aq)and H−(aq)
E)CH3CO2H(aq)and OH−(aq)
A)H+(aq)and OH−(aq)
B)CH3CO2+(aq)and OH−(aq)
C)CH3CO2+(aq)and H2O(aq)
D)CH3CO2+(aq)and H−(aq)
E)CH3CO2H(aq)and OH−(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
23
Select the two Brønsted-Lowry bases in the following equation: CH3NH2(aq)+ H2O(l)⇌ CH3NH3+(aq)+ OH−(aq)
A)CH3NH2 and H2O
B)CH3NH2 and OH−
C)H2O and OH−
D)CH3NH2 and CH3NH3+
E)CH3NH3+ and OH−
A)CH3NH2 and H2O
B)CH3NH2 and OH−
C)H2O and OH−
D)CH3NH2 and CH3NH3+
E)CH3NH3+ and OH−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
24
When ammonia, NH3, is dissolved in water, which ions are formed?
A)NH3−(aq)and OH−(aq)
B)NH3+(aq)and OH−(aq)
C)NH4+(aq)and OH−(aq)
D)NH4+(aq)and H−(aq)
E)NH2−(aq)and H+(aq)
A)NH3−(aq)and OH−(aq)
B)NH3+(aq)and OH−(aq)
C)NH4+(aq)and OH−(aq)
D)NH4+(aq)and H−(aq)
E)NH2−(aq)and H+(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
25
Select the pair that consists of a base and its conjugate acid in that order.
A)NH3/NH4+
B)HCO3−/CO32−
C)H2CO3/HCO3−
D)H3PO4/ HPO42−
E)CO32−/CO22−
A)NH3/NH4+
B)HCO3−/CO32−
C)H2CO3/HCO3−
D)H3PO4/ HPO42−
E)CO32−/CO22−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
26
The conjugate acid of HCO3− is:
A)H2CO3
B)HCO2−
C)CO32−
D)CO2
E)H3CO3+
A)H2CO3
B)HCO2−
C)CO32−
D)CO2
E)H3CO3+

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
27
Select the two Brønsted-Lowry bases in the following equation: CO32−(aq)+ H2O(l)⇌ HCO3−(aq)+ OH−(aq)
A)CO32− and H2O
B)CO32− and HCO3−
C)H2O and OH−
D)CO32− and OH−
E)H2O and HCO3−
A)CO32− and H2O
B)CO32− and HCO3−
C)H2O and OH−
D)CO32− and OH−
E)H2O and HCO3−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
28
The conjugate base of H2PO4− is:
A)HPO42−
B)H2PO3−
C)H3PO4
D)PO43−
E)H2PO4OH−
A)HPO42−
B)H2PO3−
C)H3PO4
D)PO43−
E)H2PO4OH−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
29
The conjugate acid of H2PO4− is:
A)HPO42−
B)H2PO3−
C)H3PO4
D)PO43−
E)H3PO3
A)HPO42−
B)H2PO3−
C)H3PO4
D)PO43−
E)H3PO3

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
30
Select the two Brønsted-Lowry bases in the following equation: NH3(aq)+ H2O(aq)⇌ NH4+(aq)+ OH−(aq)
A)NH3 and H2O
B)NH3 and OH−
C)H2O and OH−
D)NH3 and NH4+
E)NH4+ and OH−
A)NH3 and H2O
B)NH3 and OH−
C)H2O and OH−
D)NH3 and NH4+
E)NH4+ and OH−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
31
Select the pair that consists of a base and its conjugate acid in that order.
A)NH4+/NH3
B)HCO3−/CO32−
C)HCO3−/H2CO3
D)H3PO4/ HPO42−
E)CO32−/CO22−
A)NH4+/NH3
B)HCO3−/CO32−
C)HCO3−/H2CO3
D)H3PO4/ HPO42−
E)CO32−/CO22−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
32
Select the pair that consists of an acid and its conjugate base in that order.
A)NH3/NH4+
B)CO32−/HCO3−
C)H2CO3/HCO3−
D)HPO42−/H3PO4
E)CO32-/ CO22-
A)NH3/NH4+
B)CO32−/HCO3−
C)H2CO3/HCO3−
D)HPO42−/H3PO4
E)CO32-/ CO22-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
33
Select the pair that consists of an acid and its conjugate base in that order.
A)NH4+/NH2-
B)CO32−/HCO3−
C)CO32−/H2CO3−
D)H2PO4-/H3PO4
E)H2CO3/HCO3−
A)NH4+/NH2-
B)CO32−/HCO3−
C)CO32−/H2CO3−
D)H2PO4-/H3PO4
E)H2CO3/HCO3−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is an amphoteric substance?
A)NaCl
B)LiOH
C)KBr
D)NaHCO3
E)CH3OH
A)NaCl
B)LiOH
C)KBr
D)NaHCO3
E)CH3OH

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
35
Select the two Brønsted-Lowry acids in the following equation: CO32-(aq)+ HF(aq)⇌ HCO3-(aq)+ F-(aq)
A)CO32- and HF
B)CO32- and HCO3-
C)HF and F-
D)CO32- and F-
E)HF and HCO3-
A)CO32- and HF
B)CO32- and HCO3-
C)HF and F-
D)CO32- and F-
E)HF and HCO3-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
36
Select the pair that consists of an acid and its conjugate base in that order.
A)NH3/NH4+
B)SO42−/HSO3−
C)H2SO3/HSO3−
D)H2PO4−/H3PO4
E)CO32−/HCO32−
A)NH3/NH4+
B)SO42−/HSO3−
C)H2SO3/HSO3−
D)H2PO4−/H3PO4
E)CO32−/HCO32−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
37
Select the two Brønsted-Lowry acids in the following equation: NH3(aq)+ HF(aq)⇌ NH4+(aq)+ F−(aq)
A)NH3 and HF
B)NH3 and F−
C)HF and F−
D)NH4+ and F-
E)HF and NH4+
A)NH3 and HF
B)NH3 and F−
C)HF and F−
D)NH4+ and F-
E)HF and NH4+

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
38
When carbonate ion, CO32−, reacts with water, what are the products?
A)H+(aq)and OH−(aq)
B)HCO3(aq)and OH−(aq)
C)HCO3+(aq)and H2O(aq)
D)HCO3−(aq)and H−(aq)
E)HCO3−(aq)and OH−(aq)
A)H+(aq)and OH−(aq)
B)HCO3(aq)and OH−(aq)
C)HCO3+(aq)and H2O(aq)
D)HCO3−(aq)and H−(aq)
E)HCO3−(aq)and OH−(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
39
When fluoride ion, F−, reacts with water, what are the products?
A)H+(aq)and OH−(aq)
B)F+(aq)and OH−(aq)
C)F+(aq)and H2O(aq)
D)HF(aq)and H−(aq)
E)HF(aq)and OH−(aq)
A)H+(aq)and OH−(aq)
B)F+(aq)and OH−(aq)
C)F+(aq)and H2O(aq)
D)HF(aq)and H−(aq)
E)HF(aq)and OH−(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
40
When sodium hydroxide, NaOH, is dissolved in water, which species are present in addition to water?
A)NaOH(aq)
B)Na+(aq)and OH−(aq)
C)Na+(aq)and H3O−(aq)
D)Na+(aq)and O2H22−(aq)
E)NaO−(aq)and H3O+(aq)
A)NaOH(aq)
B)Na+(aq)and OH−(aq)
C)Na+(aq)and H3O−(aq)
D)Na+(aq)and O2H22−(aq)
E)NaO−(aq)and H3O+(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
41
Select the strong acid from the following list.
A)H3PO4(aq)
B)CH3CO2H(aq)
C)H2S(aq)
D)H2SO4(aq)
E)HF(aq)
A)H3PO4(aq)
B)CH3CO2H(aq)
C)H2S(aq)
D)H2SO4(aq)
E)HF(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
42
Select the strong acid from the following list.
A)HNO3(aq)
B)HClO3(aq)
C)HF(aq)
D)H3PO4(aq)
E)HCN(aq)
A)HNO3(aq)
B)HClO3(aq)
C)HF(aq)
D)H3PO4(aq)
E)HCN(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following ions is amphoteric?
A)Cl−
B)OH−
C)H+
D)NO3-
E)HCO3-
A)Cl−
B)OH−
C)H+
D)NO3-
E)HCO3-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
44
All of the following species are weak bases except:
A)KCN(aq)
B)NaNO2(aq)
C)LiCl(aq)
D)C6H5NH2(aq)
E)CH3CH2NH2(aq)
A)KCN(aq)
B)NaNO2(aq)
C)LiCl(aq)
D)C6H5NH2(aq)
E)CH3CH2NH2(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
45
The image shows a molecular-level representation of part of a solution after HF is dissolved in water. (Besides water, there are 6 HF molecules, 1 H3O+ ion, and 1 F− ion present in the solution.)Which of the following best describes HF? 
A)strong acid
B)strong base
C)weak acid
D)weak base
E)amphoteric

A)strong acid
B)strong base
C)weak acid
D)weak base
E)amphoteric

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is the strongest acid?
A)HF, Ka = 6.3 × 10-4
B)HCN, Ka = 6.2 × 10−10
C)HOCl, Ka = 4.0 × 10−8
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4
A)HF, Ka = 6.3 × 10-4
B)HCN, Ka = 6.2 × 10−10
C)HOCl, Ka = 4.0 × 10−8
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is an amphoteric substance?
A)NaOH
B)LiCl
C)CsBr
D)H2O
E)CH4
A)NaOH
B)LiCl
C)CsBr
D)H2O
E)CH4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
48
Match the molecular-level diagrams to each of the following compounds in aqueous solution: HCl, HF, NH3 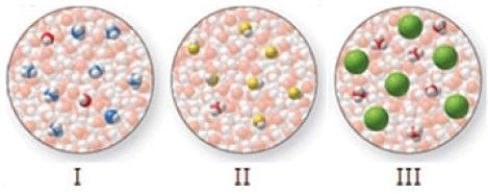
A)I = HCl, II = HF, III = NH3
B)I = HF, II = HCl, III = NH3
C)I = HF, II = NH3, III = HCl
D)I = NH3, II = HF, III = HCl
E)I = NH3, II = HCl, III = HF

A)I = HCl, II = HF, III = NH3
B)I = HF, II = HCl, III = NH3
C)I = HF, II = NH3, III = HCl
D)I = NH3, II = HF, III = HCl
E)I = NH3, II = HCl, III = HF

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
49
All of the following species are weak bases except:
A)NH3(aq)
B)Na2CO3(aq)
C)KF(aq)
D)CH3NH2(aq)
E)NH4Cl(aq)
A)NH3(aq)
B)Na2CO3(aq)
C)KF(aq)
D)CH3NH2(aq)
E)NH4Cl(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
50
Select the strong acid from the following list.
A)H2SO3(aq)
B)H2C2O4(aq)
C)H2S(aq)
D)HNO2(aq)
E)HI(aq)
A)H2SO3(aq)
B)H2C2O4(aq)
C)H2S(aq)
D)HNO2(aq)
E)HI(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
51
The H2PO4- ion is amphoteric. What are the conjugate acid and conjugate base, in that order, of H2PO4-?
A)PO43-, H2PO4-
B)H2PO4-, PO43-
C)H2PO4-, H3PO4
D)H3PO4, HPO42-
E)H3O+, PO43-
A)PO43-, H2PO4-
B)H2PO4-, PO43-
C)H2PO4-, H3PO4
D)H3PO4, HPO42-
E)H3O+, PO43-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
52
The bicarbonate ion, HCO3-, is amphoteric. What are the conjugate acid and conjugate base, in that order, of HCO3-?
A)CO32-, HCO3-
B)HCO3-, CO32-
C)CO32-, H2CO3
D)H2CO3, CO32-
E)H3O+, CO32-
A)CO32-, HCO3-
B)HCO3-, CO32-
C)CO32-, H2CO3
D)H2CO3, CO32-
E)H3O+, CO32-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
53
All of the following species are weak bases except:
A)NaCN(aq)
B)K2CO3(aq)
C)KOH(aq)
D)CH3NH2(aq)
E)NH3(aq)
A)NaCN(aq)
B)K2CO3(aq)
C)KOH(aq)
D)CH3NH2(aq)
E)NH3(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following equations represents the behavior of HCO3− as an acid?
A)HCO3−(aq)+ H3O+(aq)⇌ H2CO3(aq)+ H2O(l)
B)HCO3−(aq)+ HF(aq)⇌ H2CO3(aq)+ F−(aq)
C)HCO3−(aq)+ HCN(aq)⇌ H2CO3(aq)+ CN−(aq)
D)HCO3−(aq)+ OH−(aq)⇌ CO32−(aq)+ H2O(l)
E)HCO3−(aq)+ H2O(l)⇌ H2CO3(aq)+ OH−(aq)
A)HCO3−(aq)+ H3O+(aq)⇌ H2CO3(aq)+ H2O(l)
B)HCO3−(aq)+ HF(aq)⇌ H2CO3(aq)+ F−(aq)
C)HCO3−(aq)+ HCN(aq)⇌ H2CO3(aq)+ CN−(aq)
D)HCO3−(aq)+ OH−(aq)⇌ CO32−(aq)+ H2O(l)
E)HCO3−(aq)+ H2O(l)⇌ H2CO3(aq)+ OH−(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
55
The image shows a molecular-level representation of part of a solution after ammonia, NH3, is dissolved in water. (Besides water, there are 2 NH4+ ions, 6 NH3 molecules, and 2 OH− ions present in the solution.). Which of the following best describes NH3? 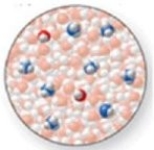
A)strong acid
B)strong base
C)weak acid
D)weak base
E)amphoteric

A)strong acid
B)strong base
C)weak acid
D)weak base
E)amphoteric

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following equations represents the behavior of H2PO4- as a base?
A)H2PO4-(aq)+ H3O+(aq)⇌ H3PO4(aq)+ H2O(l)
B)H2PO4-(aq)+ F-(aq)⇌ HPO42-(aq)+ HF(aq)
C)H2PO4-(aq)+ CN-(aq)⇌ HPO42-(aq)+ HCN(aq)
D)H2PO4-(aq)+ OH-(aq)⇌ HPO42-(aq)+ H2O(l)
E)H2PO4-(aq)+ H2O(l)⇌ H3PO4(aq)+ H3O+(aq)
A)H2PO4-(aq)+ H3O+(aq)⇌ H3PO4(aq)+ H2O(l)
B)H2PO4-(aq)+ F-(aq)⇌ HPO42-(aq)+ HF(aq)
C)H2PO4-(aq)+ CN-(aq)⇌ HPO42-(aq)+ HCN(aq)
D)H2PO4-(aq)+ OH-(aq)⇌ HPO42-(aq)+ H2O(l)
E)H2PO4-(aq)+ H2O(l)⇌ H3PO4(aq)+ H3O+(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following ions is amphoteric?
A)Br−
B)H−
C)OH+
D)SO42−
E)HPO42−
A)Br−
B)H−
C)OH+
D)SO42−
E)HPO42−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following equations represents the behavior of H2PO4- as an acid?
A)H2PO4-(aq)+ H3O+(aq)⇌ H3PO4(aq)+ H2O(l)
B)H2PO4-(aq)+ HF(aq)⇌ H3PO4(aq)+ F-(aq)
C)H2PO4-(aq)+ HCN(aq)⇌ H3PO4(aq)+ CN-(aq)
D)H2PO4-(aq)+ OH-(aq)⇌ HPO42-(aq)+ H2O(l)
E)H2PO4-(aq)+ H2O(l)⇌ H3PO4(aq)+ OH-(aq)
A)H2PO4-(aq)+ H3O+(aq)⇌ H3PO4(aq)+ H2O(l)
B)H2PO4-(aq)+ HF(aq)⇌ H3PO4(aq)+ F-(aq)
C)H2PO4-(aq)+ HCN(aq)⇌ H3PO4(aq)+ CN-(aq)
D)H2PO4-(aq)+ OH-(aq)⇌ HPO42-(aq)+ H2O(l)
E)H2PO4-(aq)+ H2O(l)⇌ H3PO4(aq)+ OH-(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
59
The image shows a molecular-level representation of part of a solution after HCl is dissolved in water. What best describes this image? (Besides water, there are 6 H3O+ ions and 6 Cl− ions present in the solution.)Which of the following best describes HCl? 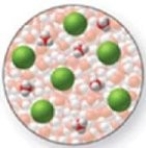
A)strong acid
B)strong base
C)weak acid
D)weak base
E)amphoteric

A)strong acid
B)strong base
C)weak acid
D)weak base
E)amphoteric

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following equations represents the behavior of HCO3− as a base?
A)HCO3−(aq)+ H3O+(aq)⇌ H2CO3(aq)+ H2O(l)
B)HCO3−(aq)+ F−(aq)⇌ CO32−(aq)+ HF(aq)
C)HCO3−(aq)+ CN−(aq)⇌ CO32−(aq)+ HCN(aq)
D)HCO3−(aq)+ OH−(aq)⇌ CO32−(aq)+ H2O(l)
E)HCO3−(aq)+ H2O(l)⇌ H2CO3(aq)+ H3O+(aq)
A)HCO3−(aq)+ H3O+(aq)⇌ H2CO3(aq)+ H2O(l)
B)HCO3−(aq)+ F−(aq)⇌ CO32−(aq)+ HF(aq)
C)HCO3−(aq)+ CN−(aq)⇌ CO32−(aq)+ HCN(aq)
D)HCO3−(aq)+ OH−(aq)⇌ CO32−(aq)+ H2O(l)
E)HCO3−(aq)+ H2O(l)⇌ H2CO3(aq)+ H3O+(aq)

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
61
The Ka for formic acid is 1.8 × 10-4. Which of the following statements best describes the pH of a 0.0010 M solution of formic acid?
A)The pH is greater than 0 but less than 3.
B)The pH is exactly 3.
C)The pH is greater than 3 but less than 7.
D)The pH is exactly 7.
E)The pH is greater than 7 but less than 11.
A)The pH is greater than 0 but less than 3.
B)The pH is exactly 3.
C)The pH is greater than 3 but less than 7.
D)The pH is exactly 7.
E)The pH is greater than 7 but less than 11.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
62
List the species present in order of increasing concentration in a 0.1 M solution of H2Se.
A)H2Se < HSe- < Se2−
B)H2Se < Se2− < HSe−
C)Se2- < HSe- < H2Se
D)HSe- < Se2- < H2Se
E)Se2− < H2Se < HSe−
A)H2Se < HSe- < Se2−
B)H2Se < Se2− < HSe−
C)Se2- < HSe- < H2Se
D)HSe- < Se2- < H2Se
E)Se2− < H2Se < HSe−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
63
Select the solution below that is the most acidic.
A)[OH-] = 1.0 × 10-4 M
B)[OH-] = 1.0 × 10-5 M
C)[OH-] = 1.0 × 10-7 M
D)[OH-] = 1.0 × 10-9 M
E)[OH-] = 1.0 × 10-11 M
A)[OH-] = 1.0 × 10-4 M
B)[OH-] = 1.0 × 10-5 M
C)[OH-] = 1.0 × 10-7 M
D)[OH-] = 1.0 × 10-9 M
E)[OH-] = 1.0 × 10-11 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
64
Select the solution below that is the most basic.
A)[H3O+] = 1.0 × 10-4 M
B)[H3O+] = 1.0 × 10-6 M
C)[H3O+] = 1.0 × 10-7 M
D)[H3O+] = 1.0 × 10-8 M
E)[H3O+] = 1.0 × 10-10 M
A)[H3O+] = 1.0 × 10-4 M
B)[H3O+] = 1.0 × 10-6 M
C)[H3O+] = 1.0 × 10-7 M
D)[H3O+] = 1.0 × 10-8 M
E)[H3O+] = 1.0 × 10-10 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
65
Select the solution below that is the most basic.
A)[OH-] = 1.0 × 10-4 M
B)[OH-] = 1.0 × 10-5 M
C)[OH-] = 1.0 × 10-7 M
D)[OH-] = 1.0 × 10-9 M
E)[OH-] = 1.0 × 10-11 M
A)[OH-] = 1.0 × 10-4 M
B)[OH-] = 1.0 × 10-5 M
C)[OH-] = 1.0 × 10-7 M
D)[OH-] = 1.0 × 10-9 M
E)[OH-] = 1.0 × 10-11 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following ionizes to the greatest extent?
A)HNO2, Ka = 5.6 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)NH4+, KKa = 5.6 × 10−10
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4
A)HNO2, Ka = 5.6 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)NH4+, KKa = 5.6 × 10−10
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
67
List the species present in order of increasing concentration in a 0.1 M solution of H2S.
A)H2S < HS- < S2-
B)H2S < S2- < HS-
C)S2- < HS- < H2S
D)HS- < S2- < H2S
E)S2- < H2S < HS-
A)H2S < HS- < S2-
B)H2S < S2- < HS-
C)S2- < HS- < H2S
D)HS- < S2- < H2S
E)S2- < H2S < HS-

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following ionizes to the greatest extent?
A)HF, Ka = 6.3 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)HOCl, Ka = 4.0 × 10−8
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4
A)HF, Ka = 6.3 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)HOCl, Ka = 4.0 × 10−8
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
69
Given 0.10 M solutions of the following acids, which contains the highest concentration of H3O+?
A)HF, Ka = 6.3 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)HOCl, Ka = 4.0 × 10−8
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4
A)HF, Ka = 6.3 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)HOCl, Ka = 4.0 × 10−8
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
70
Select the solution below that is the most acidic.
A)[H3O+] = 1.0 × 10-4 M
B)[H3O+] = 1.0 × 10-6 M
C)[H3O+] = 1.0 × 10-7 M
D)[H3O+] = 1.0 × 10-8 M
E)[H3O+] = 1.0 × 10-10 M
A)[H3O+] = 1.0 × 10-4 M
B)[H3O+] = 1.0 × 10-6 M
C)[H3O+] = 1.0 × 10-7 M
D)[H3O+] = 1.0 × 10-8 M
E)[H3O+] = 1.0 × 10-10 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
71
Rank the following 0.100 M solutions in order of increasing H3O+ concentration: HF, Ka = 6.3 × 10−4; HCN, Ka = 6.2 × 10−10; HOCl, Ka = 4.0 × 10−8
A)HF < HCN < HOCl
B)HF < HOCl < HCN
C)HOCl < HF < HCN
D)HOCl < HCN < HF
E)HCN < HOCl < HF
A)HF < HCN < HOCl
B)HF < HOCl < HCN
C)HOCl < HF < HCN
D)HOCl < HCN < HF
E)HCN < HOCl < HF

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
72
The Ka for acetic acid is 1.8 × 10-5. Which of the following statements best describes the pH of a 0.010 M solution of acetic acid?
A)The pH is greater than 0 but less than 2.
B)The pH is exactly 2.
C)The pH is greater than 2 but less than 7.
D)The pH is exactly 7.
E)The pH is greater than 7 but less than 12.
A)The pH is greater than 0 but less than 2.
B)The pH is exactly 2.
C)The pH is greater than 2 but less than 7.
D)The pH is exactly 7.
E)The pH is greater than 7 but less than 12.

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
73
Given 0.10 M solutions of the following acids, which contains the highest concentration of H3O+?
A)HNO2, Ka = 5.6 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)NH4+, Ka = 5.6 × 10−10
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4
A)HNO2, Ka = 5.6 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)NH4+, Ka = 5.6 × 10−10
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
74
Given 0.10 M solutions of the following acids, which contains the lowest concentration of H3O+?
A)HF, Ka = 6.3 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)HOCl, Ka = 4.0 × 10−8
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4
A)HF, Ka = 6.3 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)HOCl, Ka = 4.0 × 10−8
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
75
Rank the following 0.100 M solutions in order of increasing H3O+ concentration: HOCl, Ka = 4.0 × 10−8; HCN, Ka = 6.2 × 10−10; NH4+, Ka = 5.6 × 10−10
A)HOCl < HCN < NH4+
B)HOCl < NH4+ < HCN
C)HCN < NH4+ < HOCl
D)HCN < HOCl < NH4+
E)NH4+ < HCN < HOCl
A)HOCl < HCN < NH4+
B)HOCl < NH4+ < HCN
C)HCN < NH4+ < HOCl
D)HCN < HOCl < NH4+
E)NH4+ < HCN < HOCl

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is the strongest acid?
A)HOCl, Ka = 4.0 × 10−8
B)HCN, Ka = 6.2 × 10−10
C)NH4+, Ka = 5.6 × 10−10
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4
A)HOCl, Ka = 4.0 × 10−8
B)HCN, Ka = 6.2 × 10−10
C)NH4+, Ka = 5.6 × 10−10
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is the strongest acid?
A)HNO2, Ka = 5.6 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)NH4+, Ka = 5.6 × 10−10
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4
A)HNO2, Ka = 5.6 × 10−4
B)HCN, Ka = 6.2 × 10−10
C)NH4+, Ka = 5.6 × 10−10
D)CH3CO2H, Ka = 1.8 × 10−5
E)HCO2H, Ka = 1.8 × 10−4

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
78
Rank the following 0.100 M solutions in order of increasing H3O+ concentration: HCN, Ka = 6.2 × 10-10; NH4+, Ka = 5.6 × 10-10; CH3CO2H, Ka = 1.8 × 10-5
A)HCN < NH4+ < CH3CO2H
B)HCN < CH3CO2H < NH4+
C)CH3CO2H < NH4+ < HCN
D)CH3CO2H < HCN < NH4+
E)NH4+ < HCN < CH3CO2H
A)HCN < NH4+ < CH3CO2H
B)HCN < CH3CO2H < NH4+
C)CH3CO2H < NH4+ < HCN
D)CH3CO2H < HCN < NH4+
E)NH4+ < HCN < CH3CO2H

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
79
List the species present in order of increasing concentration in a 0.1 M solution of H2C2O4.
A)H2C2O4 < HC2O4− < C2O42−
B)H2C2O4 < C2O42− < HC2O4−
C)C2O42− < HC2O4− < H2C2O4
D)HC2O4− < C2O42− < H2C2O4
E)C2O42− < H2C2O4 < HC2O4−
A)H2C2O4 < HC2O4− < C2O42−
B)H2C2O4 < C2O42− < HC2O4−
C)C2O42− < HC2O4− < H2C2O4
D)HC2O4− < C2O42− < H2C2O4
E)C2O42− < H2C2O4 < HC2O4−

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck
80
Select the solution below that is the most acidic.
A)[OH-] = 1.0 × 10-4 M
B)[OH-] = 1.0 × 10-5 M
C)[H3O+] = 1.0 × 10-6 M
D)[H3O+] = 1.0 × 10-8 M
E)[H3O+] = 1.0 × 10-10 M
A)[OH-] = 1.0 × 10-4 M
B)[OH-] = 1.0 × 10-5 M
C)[H3O+] = 1.0 × 10-6 M
D)[H3O+] = 1.0 × 10-8 M
E)[H3O+] = 1.0 × 10-10 M

Unlock Deck
Unlock for access to all 137 flashcards in this deck.
Unlock Deck
k this deck


