Deck 9: The Gaseous State
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/121
Play
Full screen (f)
Deck 9: The Gaseous State
1
Which of the following statements is incorrect? (Assume a fixed amount of gas under constant temperature conditions.)
A)Boyle's law says that the volume occupied by a gas is inversely proportional to its pressure.
B)If the volume of a container of gas is halved, the pressure will be doubled.
C)If the volume of a container of gas is tripled, the pressure will decrease by a factor of three.
D)When the volume of a container is increased, the distance between the gas particles decreases.
E)If the pressure on a gas sample is quadrupled, the volume will decrease by a factor of four.
A)Boyle's law says that the volume occupied by a gas is inversely proportional to its pressure.
B)If the volume of a container of gas is halved, the pressure will be doubled.
C)If the volume of a container of gas is tripled, the pressure will decrease by a factor of three.
D)When the volume of a container is increased, the distance between the gas particles decreases.
E)If the pressure on a gas sample is quadrupled, the volume will decrease by a factor of four.
When the volume of a container is increased, the distance between the gas particles decreases.
2
If a 2.75 L gas sample is held at a constant temperature, and its pressure is changed from 750.0 torr to 360.0 torr, what will the final volume be?
A)2.75 L
B)5.73 L
C)1.32 L
D)1.41 L
E)2.92 L
A)2.75 L
B)5.73 L
C)1.32 L
D)1.41 L
E)2.92 L
5.73 L
3
Which of the following is not a property of gases?
A)Gases are compressible.
B)Gases expand to fill their containers.
C)The density of a gas is similar to that of a liquid.
D)Gas particles move about rapidly.
E)If one property, such as pressure or volume, or a gas changes, one or more other properties must change as well.
A)Gases are compressible.
B)Gases expand to fill their containers.
C)The density of a gas is similar to that of a liquid.
D)Gas particles move about rapidly.
E)If one property, such as pressure or volume, or a gas changes, one or more other properties must change as well.
The density of a gas is similar to that of a liquid.
4
Which of the following statements regarding gas pressure is correct?
A)Gas pressure is commonly measured in units of pounds per square foot.
B)The pressure of air on the outside of a metal can with a low internal pressure is sufficient to crush the can.
C)If the volume of a container filled with a gas increases at constant temperature, the pressure will also increase.
D)If the temperature of a gas sample increases while the gas is held at a constant volume, the pressure will decrease.
E)When the volume of a container of a gas increases, the gas particles will stay together in the bottom of the container.
A)Gas pressure is commonly measured in units of pounds per square foot.
B)The pressure of air on the outside of a metal can with a low internal pressure is sufficient to crush the can.
C)If the volume of a container filled with a gas increases at constant temperature, the pressure will also increase.
D)If the temperature of a gas sample increases while the gas is held at a constant volume, the pressure will decrease.
E)When the volume of a container of a gas increases, the gas particles will stay together in the bottom of the container.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
5
Which physical characteristic does not apply to a gas?
A)It can be compressed.
B)Its molecules are separated by large distances.
C)It occupies the total volume of its container.
D)It has a high density.
E)It can be formed by the evaporation of its liquid state.
A)It can be compressed.
B)Its molecules are separated by large distances.
C)It occupies the total volume of its container.
D)It has a high density.
E)It can be formed by the evaporation of its liquid state.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
6
Convert 689 torr to atm.
A)0.689 atm
B)0.760 atm
C)0.906 atm
D)1.10 atm
E)9.98 x 107 atm
A)0.689 atm
B)0.760 atm
C)0.906 atm
D)1.10 atm
E)9.98 x 107 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
7
The "Before" image in the figure shows the initial condition of a gas at a certain temperature in a container with a movable piston. Which of the images represents the condition of the gas when the temperature of the gas is increased, and the external pressure is held constant? 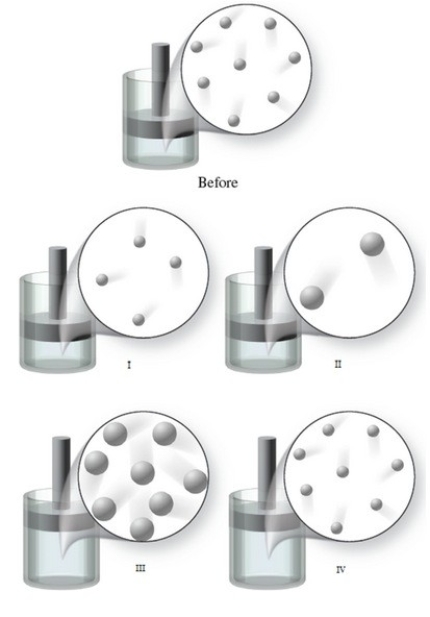
A)image I
B)image II
C)image III
D)image IV
E)None of the images

A)image I
B)image II
C)image III
D)image IV
E)None of the images

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
8
If a 1.50 L gas sample is held at a constant temperature, and its pressure is changed from 2.30 atm to 3.60 atm, what will the final volume be?
A)1.50 L
B)3.45 L
C)0.958 L
D)8.28 L
E)0.828 L
A)1.50 L
B)3.45 L
C)0.958 L
D)8.28 L
E)0.828 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
9
Convert 1.13 atm to torr.
A)1.13 x 103 torr
B)1.14 x 105 torr
C)859 torr
D)1.16 x 10-3 torr
E)1.49 x 10-3 torr
A)1.13 x 103 torr
B)1.14 x 105 torr
C)859 torr
D)1.16 x 10-3 torr
E)1.49 x 10-3 torr

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is incorrect?
A)Warm air is less dense than cold air.
B)Warm air rises above cold air.
C)When gases are heated, they expand.
D)The properties of gases are very similar to the properties of liquids.
E)Many properties of gases are the same, regardless of the chemical makeup of the gases.
A)Warm air is less dense than cold air.
B)Warm air rises above cold air.
C)When gases are heated, they expand.
D)The properties of gases are very similar to the properties of liquids.
E)Many properties of gases are the same, regardless of the chemical makeup of the gases.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
11
According to kinetic-molecular theory, in which of the following gases will the average speed of the molecules be the highest at 400°C?
A)HF
B)Cl2
C)H2O
D)SF6
E)SO2
A)HF
B)Cl2
C)H2O
D)SF6
E)SO2

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
12
Convert 986 mm Hg to Pa.
A)1.30 Pa
B)1.01 x 103 Pa
C)1.31 x 105 Pa
D)9.99 x 107 Pa
E)7.49 x 105 Pa
A)1.30 Pa
B)1.01 x 103 Pa
C)1.31 x 105 Pa
D)9.99 x 107 Pa
E)7.49 x 105 Pa

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is not a property of gases?
A)Gases consist of particles that are relatively far apart.
B)Gases can be compressed in a container.
C)Gas particles collide with one another.
D)When gas particles collide, attractive forces keep them attached to one another.
E)When gases are stored under pressure, their densities increase.
A)Gases consist of particles that are relatively far apart.
B)Gases can be compressed in a container.
C)Gas particles collide with one another.
D)When gas particles collide, attractive forces keep them attached to one another.
E)When gases are stored under pressure, their densities increase.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
14
Convert 0.157 atm to mm Hg.
A)119 mm Hg
B)8.38 x 10-3 mm Hg
C)2.07 x 10-4 mm Hg
D)0.157 mm Hg
E)1.59 x 104 mm Hg
A)119 mm Hg
B)8.38 x 10-3 mm Hg
C)2.07 x 10-4 mm Hg
D)0.157 mm Hg
E)1.59 x 104 mm Hg

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
15
The figure shows a "Before" image of gas atoms at a certain temperature. If the volume of the container increases, and the temperature remains constant, which of the images represents the final condition of the gas? 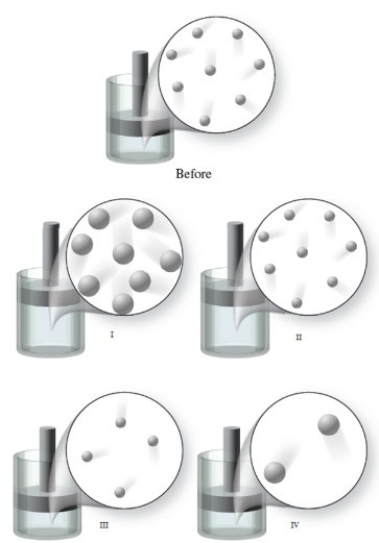
A)image I
B)image II
C)image III
D)image IV
E)None of the images

A)image I
B)image II
C)image III
D)image IV
E)None of the images

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
16
Convert 1.28 atm to mm Hg.
A)1.28 x 103 mm Hg
B)1.30 x 105 mm Hg
C)1.68 x 10-3 mm Hg
D)972.8 mm Hg
E)1.03 x 10-3 mm Hg
A)1.28 x 103 mm Hg
B)1.30 x 105 mm Hg
C)1.68 x 10-3 mm Hg
D)972.8 mm Hg
E)1.03 x 10-3 mm Hg

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
17
Convert 705 torr to atm.
A)7.01 x 107 atm
B)0.705 atm
C)0.928 atm
D)1.08 atm
E)5.36 x 105 atm
A)7.01 x 107 atm
B)0.705 atm
C)0.928 atm
D)1.08 atm
E)5.36 x 105 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
18
If the initial pressure of a 2.00 L gas sample is 2.50 atm, what will the pressure be if the volume is changed to 3.00 L at constant temperature?
A)1.50 atm
B)1.67 atm
C)15.0 atm
D)2.40 atm
E)0.600 atm
A)1.50 atm
B)1.67 atm
C)15.0 atm
D)2.40 atm
E)0.600 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
19
Convert 723 torr to atm.
A)7.32 x 107 atm
B)0.723 atm
C)0.760 atm
D)0.951 atm
E)1.05 atm
A)7.32 x 107 atm
B)0.723 atm
C)0.760 atm
D)0.951 atm
E)1.05 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
20
Convert 6.25 x 104 Pa to mm Hg.
A)0.617 mm Hg
B)469 mm Hg
C)4.75 x 107 mm Hg
D)82.2 mm Hg
E)625 mm Hg
A)0.617 mm Hg
B)469 mm Hg
C)4.75 x 107 mm Hg
D)82.2 mm Hg
E)625 mm Hg

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
21
Given a fixed amount of gas in a rigid container (no change in volume), what temperature will the gas have to be changed to if the pressure is initially 3.50 atm at 22.0°C, and the desired final pressure is 1.75 atm?
A)−126°C
B)11.0°C
C)44.0°C
D)148°C
E)−148°C
A)−126°C
B)11.0°C
C)44.0°C
D)148°C
E)−148°C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
22
Given a fixed amount of gas in a rigid container (no change in volume), what pressure will the gas exert if the pressure is initially 2.50 atm at 22.0°C, and the temperature is changed to 66.0°C?
A)7.50 atm
B)2.87 atm
C)0.833 atm
D)2.50 atm
E)3.75 atm
A)7.50 atm
B)2.87 atm
C)0.833 atm
D)2.50 atm
E)3.75 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
23
Given a fixed amount of gas in a rigid container (no change in volume), what temperature will the gas have to be changed to if the pressure is initially 1.50 atm at 42.0°C, and the desired final pressure is 3.00 atm?
A)84.0°C
B)21.0°C
C)630°C
D)357°C
E)273°C
A)84.0°C
B)21.0°C
C)630°C
D)357°C
E)273°C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
24
A sample of gas initially occupies 5.50 L at a pressure of 0.750 atm at 13.0°C. What will the pressure be if the temperature is changed to 22.5°C, and the volume is changed to 1.50 L?
A)6.39 atm
B)11.4 atm
C)0.211 atm
D)2.84 atm
E)1.59 atm
A)6.39 atm
B)11.4 atm
C)0.211 atm
D)2.84 atm
E)1.59 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
25
Given a fixed amount of gas held at constant pressure, calculate the volume it would occupy if a 3.50 L sample were cooled from 90.0oC to 30.0oC.
A)1.17 L
B)10.5 L
C)4.19 L
D)2.92 L
E)1.75 L
A)1.17 L
B)10.5 L
C)4.19 L
D)2.92 L
E)1.75 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
26
A sample of gas initially occupies 4.25 L at a pressure of 0.850 atm at 23.0°C. What will the volume be if the temperature is changed to 11.5°C, and the pressure is changed to 1.50 atm?
A)2.31 L
B)1.20 L
C)4.82 L
D)2.50 L
E)7.21 L
A)2.31 L
B)1.20 L
C)4.82 L
D)2.50 L
E)7.21 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
27
Given a fixed amount of gas held at constant pressure, calculate the volume it would occupy if a 2.00 L sample were cooled from 60.0oC to 30.0oC.
A)1.00 L
B)4.00 L
C)2.20 L
D)0.455 L
E)1.82 L
A)1.00 L
B)4.00 L
C)2.20 L
D)0.455 L
E)1.82 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements is incorrect? (Assume that pressure and amount of gas are constant.)
A)Charles's law says that volume is directly proportional to temperature.
B)If the absolute temperature of a gas doubles, then the volume of the gas will double.
C)If the volume of a gas is halved, then the absolute temperature of the gas will be halved also.
D)If the temperature of a gas sample decreases from 50oC to 25oC, the pressure will be halved.
E)When a gas is cooled, the particles move more slowly.
A)Charles's law says that volume is directly proportional to temperature.
B)If the absolute temperature of a gas doubles, then the volume of the gas will double.
C)If the volume of a gas is halved, then the absolute temperature of the gas will be halved also.
D)If the temperature of a gas sample decreases from 50oC to 25oC, the pressure will be halved.
E)When a gas is cooled, the particles move more slowly.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
29
Given a fixed amount of gas held at constant pressure, calculate the temperature to which the gas would have to be changed if a 1.75 L sample at 23.0°C were to have a final volume of 3.50 L.
A)46.0°C
B)89.5°C
C)169°C
D)319°C
E)592°C
A)46.0°C
B)89.5°C
C)169°C
D)319°C
E)592°C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
30
If the initial pressure of a 3.00 L gas sample is 2.50 atm, what will the pressure be if the volume is changed to 4.00 L at constant temperature?
A)5.50 atm
B)2.50 atm
C)7.50 atm
D)1.88 atm
E)0.533 atm
A)5.50 atm
B)2.50 atm
C)7.50 atm
D)1.88 atm
E)0.533 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
31
If the temperature of a gas at a constant pressure is increased, the volume will
A)become smaller because of fewer collisions with the sides of the container.
B)become larger because of fewer collisions with the sides of the container.
C)become smaller because of more collisions with the sides of the container.
D)become larger because of more collisions with the sides of the container.
E)stay the same because temperature has no effect on pressure.
A)become smaller because of fewer collisions with the sides of the container.
B)become larger because of fewer collisions with the sides of the container.
C)become smaller because of more collisions with the sides of the container.
D)become larger because of more collisions with the sides of the container.
E)stay the same because temperature has no effect on pressure.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
32
A given mass of gas in a rigid container is heated from 100°C to 500°C. Which of the following best describes what will happen to the pressure of the gas?
A)The pressure will remain the same.
B)The pressure will decrease by a factor of five.
C)The pressure will increase by a factor of five.
D)The pressure will increase by a factor less than five.
E)The pressure will increase by a factor greater than five.
A)The pressure will remain the same.
B)The pressure will decrease by a factor of five.
C)The pressure will increase by a factor of five.
D)The pressure will increase by a factor less than five.
E)The pressure will increase by a factor greater than five.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
33
Given a fixed amount of gas held at constant pressure, calculate the temperature to which the gas would have to be changed if a 3.50 L sample at 23.0°C were to have a final volume of 1.50 L.
A)2.0°C
B)127°C
C)−146°C
D)9.8°C
E)43.2°C
A)2.0°C
B)127°C
C)−146°C
D)9.8°C
E)43.2°C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
34
Charles's Law states that the volume of a gas held at constant pressure is directly proportional to the absolute temperature. Which of the following is a consequence of Charles's Law?
A)Oxygen cylinders are often used by climbers on Mt. Everest.
B)Underwater divers often use air cylinders.
C)Gases can be condensed to liquids at certain temperatures and pressures.
D)A sealed balloon will rise if the air in it is heated.
E)Application of sufficient pressure to carbon dioxide gas produces solid dry ice.
A)Oxygen cylinders are often used by climbers on Mt. Everest.
B)Underwater divers often use air cylinders.
C)Gases can be condensed to liquids at certain temperatures and pressures.
D)A sealed balloon will rise if the air in it is heated.
E)Application of sufficient pressure to carbon dioxide gas produces solid dry ice.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
35
A gas in a closed container with constant volume is heated from room temperature to 100°C. According to the kinetic molecular theory, the
A)average velocity of the molecules will increase.
B)gas will increase in weight.
C)individual molecules of the gas will increase their size.
D)average distance between molecules will increase.
E)pressure on the sides of the container will decrease.
A)average velocity of the molecules will increase.
B)gas will increase in weight.
C)individual molecules of the gas will increase their size.
D)average distance between molecules will increase.
E)pressure on the sides of the container will decrease.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
36
A sample of gas initially occupies 3.35 L at a pressure of 0.950 atm at 13.0°C. What will the volume be if the temperature is changed to 22.5°C, and the pressure is changed to 1.05 atm?
A)5.13 L
B)3.13 L
C)1.79 L
D)2.93 L
E)3.58 L
A)5.13 L
B)3.13 L
C)1.79 L
D)2.93 L
E)3.58 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
37
For which of the following changes is it not clear whether the volume of a particular sample of an ideal gas will increase or decrease?
A)increase the temperature and increase the pressure
B)decrease the temperature and increase the pressure
C)increase the temperature and decrease the pressure
D)increase the temperature and keep the pressure constant
E)keep temperature constant and decrease the pressure
A)increase the temperature and increase the pressure
B)decrease the temperature and increase the pressure
C)increase the temperature and decrease the pressure
D)increase the temperature and keep the pressure constant
E)keep temperature constant and decrease the pressure

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
38
If the volume of a sample of gas is held constant, while the temperature is decreased, the pressure will
A)become higher because of fewer collisions with the container.
B)become lower because of fewer collisions with the container.
C)become lower because of more collisions with the container.
D)become higher because of more collisions with the container.
E)stay the same because temperature has no effect on pressure.
A)become higher because of fewer collisions with the container.
B)become lower because of fewer collisions with the container.
C)become lower because of more collisions with the container.
D)become higher because of more collisions with the container.
E)stay the same because temperature has no effect on pressure.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
39
Given a fixed amount of gas in a rigid container (no change in volume), what pressure will the gas exert if the pressure is initially 1.50 atm at 22.0°C, and the temperature is changed to 11.0°C?
A)0.750 atm
B)3.00 atm
C)1.56 atm
D)1.44 atm
E)301 atm
A)0.750 atm
B)3.00 atm
C)1.56 atm
D)1.44 atm
E)301 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
40
The pressure-volume relationship expressed by Boyle's law can be explained by the kinetic molecular theory as follows:
A)When a gas is subjected to more pressure, more of the gas dissolves in a liquid.
B)When the volume of a gas is decreased, its molecules become closer together, causing more frequent collisions with the walls of the container.
C)The volume of a gas decreases as the pressure applied to the gas increases.
D)When the volume is decreased, the increased temperature causes the molecules to move faster and to hit the walls of the container more frequently.
E)The pressure of a gas results from its molecules being so close to one another that they cause the container walls to bulge.
A)When a gas is subjected to more pressure, more of the gas dissolves in a liquid.
B)When the volume of a gas is decreased, its molecules become closer together, causing more frequent collisions with the walls of the container.
C)The volume of a gas decreases as the pressure applied to the gas increases.
D)When the volume is decreased, the increased temperature causes the molecules to move faster and to hit the walls of the container more frequently.
E)The pressure of a gas results from its molecules being so close to one another that they cause the container walls to bulge.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
41
Assuming that all of the gas in the tank in the figure is used to fill balloons with an average volume of 2.0 L of helium, approximately how many balloons could be filled at STP? 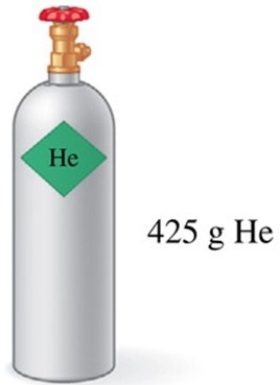
A)about 45 balloons
B)about 106 balloons
C)about 210 balloons
D)about 850 balloons
E)about 1200 balloons

A)about 45 balloons
B)about 106 balloons
C)about 210 balloons
D)about 850 balloons
E)about 1200 balloons

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
42
A sample of gas initially occupies 2.50 L at a pressure of 0.900 atm at 22.0°C. What will the pressure be if the temperature is changed to 56.5°C, and the volume is changed to 1.50 L?
A)0.584 atm
B)3.85 atm
C)1.68 atm
D)1.34 atm
E)3.77 atm
A)0.584 atm
B)3.85 atm
C)1.68 atm
D)1.34 atm
E)3.77 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the number of moles in 75.0 g of N2, and the volume that it would occupy at 50.0°C and 1.50 atm.
A)2.68 mol, 60.0 L
B)2.68 mol, 47.4 L
C)2.10 x 103 mol, 93.7 L
D)2.10 x 103 mol, 4.71 x 104 L
E)5.36 mol, 121 L
A)2.68 mol, 60.0 L
B)2.68 mol, 47.4 L
C)2.10 x 103 mol, 93.7 L
D)2.10 x 103 mol, 4.71 x 104 L
E)5.36 mol, 121 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the number of moles and the mass of gas in 3.40 L of He at STP.
A)0.152 mol, 0.608 g
B)76.2 mol, 305 g
C)0.152 mol, 0.0379 g
D)76.2 mol, 19.0 g
E)1.00 mol, 4.00 g
A)0.152 mol, 0.608 g
B)76.2 mol, 305 g
C)0.152 mol, 0.0379 g
D)76.2 mol, 19.0 g
E)1.00 mol, 4.00 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
45
A sample of gas initially occupies 2.50 L at a pressure of 905 torr at 22.0°C. What will the temperature be if the pressure is changed to 2.00 atm, and the volume is changed to 1.50 L?
A)22.2°C
B)554°C
C)1.09°C
D)271°C
E)24.5°C
A)22.2°C
B)554°C
C)1.09°C
D)271°C
E)24.5°C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
46
The two balloons shown in the figure have the same volume, and are at the same temperature and pressure. Which balloon has the greater number of gas particles, and why? 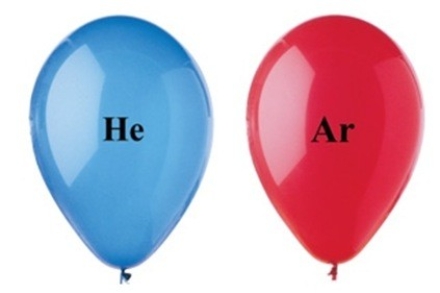
A)The Ar balloon has fewer molecules than the He balloon.
B)The density of the Ar balloon is greater than the density of the He balloon.
C)The mass of the He balloon is greater than the mass of the Ar balloon.
D)The CO2 balloon is more buoyant than the O2 balloon.
E)The He balloon has fewer moles than the He balloon.

A)The Ar balloon has fewer molecules than the He balloon.
B)The density of the Ar balloon is greater than the density of the He balloon.
C)The mass of the He balloon is greater than the mass of the Ar balloon.
D)The CO2 balloon is more buoyant than the O2 balloon.
E)The He balloon has fewer moles than the He balloon.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate the number of moles and the mass of gas in 2.50 L of Ar at STP.
A)56.0 mol, 784 g
B)56.0 mol, 2.00 g
C)0.112 mol, 0.00279 g
D)0.112 mol, 4.47 g
E)1.00 mol, 40.0 g
A)56.0 mol, 784 g
B)56.0 mol, 2.00 g
C)0.112 mol, 0.00279 g
D)0.112 mol, 4.47 g
E)1.00 mol, 40.0 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
48
The number of molecules in equal volumes of two different gases under the same conditions of temperature and pressure are
A)widely different.
B)extremely numerous.
C)impossible to compare without specific data.
D)equal.
E)greater for the molecule with the greater molar mass.
A)widely different.
B)extremely numerous.
C)impossible to compare without specific data.
D)equal.
E)greater for the molecule with the greater molar mass.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
49
A liter container of CO2 and a liter container of H2 are both at 25°C and 1 atm pressure. Which of the following statements about these gas samples is true?
A)The CO2 and H2 molecules have the same average velocity.
B)There are more H2 molecules than CO2 molecules.
C)The average kinetic energy of the CO2 molecules is greater than that of the H2 molecules.
D)The CO2 molecules on average are moving more slowly than the H2 molecules.
E)The masses of the two gas samples are equal.
A)The CO2 and H2 molecules have the same average velocity.
B)There are more H2 molecules than CO2 molecules.
C)The average kinetic energy of the CO2 molecules is greater than that of the H2 molecules.
D)The CO2 molecules on average are moving more slowly than the H2 molecules.
E)The masses of the two gas samples are equal.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
50
Calculate the number of moles in 55 g of an unknown gas, and the volume that it would occupy at STP.
A)2.0 mol, 45 L
B)2.0 mol, 0.089 L
C)1.5 x 103 mol, 67 L
D)1.5 x 103 mol, 3.4 x 104 L
E)not enough information
A)2.0 mol, 45 L
B)2.0 mol, 0.089 L
C)1.5 x 103 mol, 67 L
D)1.5 x 103 mol, 3.4 x 104 L
E)not enough information

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
51
Calculate the number of moles in 75 g of O2, and the volume that it would occupy at STP.
A)2.3 mol, 52 L
B)2.3 mol, 0.10 L
C)2.4 x 103 mol, 107 L
D)2.4 x 103 mol, 5.4 x 104 L
E)4.7 mol, 52 L
A)2.3 mol, 52 L
B)2.3 mol, 0.10 L
C)2.4 x 103 mol, 107 L
D)2.4 x 103 mol, 5.4 x 104 L
E)4.7 mol, 52 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
52
Calculate the number of moles and the mass of gas in 5.25 L of N2 at STP.
A)0.234 mol, 0.00836 g
B)0.234 mol, 3.28 g
C)0.234 mol, 6.56 g
D)118 mol, 4.20 g
E)118 mol, 1652 g
A)0.234 mol, 0.00836 g
B)0.234 mol, 3.28 g
C)0.234 mol, 6.56 g
D)118 mol, 4.20 g
E)118 mol, 1652 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
53
A sample of gas initially occupies 3.50 L at a pressure of 795 torr at 32.0°C. What will the temperature be if the pressure is changed to 4.00 atm, and the volume is changed to 1.50 L?
A)0.658°C
B)225°C
C)244°C
D)52.2°C
E)3.60°C
A)0.658°C
B)225°C
C)244°C
D)52.2°C
E)3.60°C

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
54
Calculate the number of moles in 7.5 g of Ar, and the volume that it would occupy at STP.
A)0.19 mol, 0.0085 L
B)0.19 mol, 4.2 L
C)3.0 x 102 mol, 13 L
D)3.0 x 102 mol, 6.7 x 103 L
E)1.0 mol, 22 L
A)0.19 mol, 0.0085 L
B)0.19 mol, 4.2 L
C)3.0 x 102 mol, 13 L
D)3.0 x 102 mol, 6.7 x 103 L
E)1.0 mol, 22 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the number of moles and the mass of gas in 10.2 L of O2 at STP.
A)0.455 mol, 7.28 g
B)0.455 mol, 14.6 g
C)228 mol, 7.14 g
D)228 mol, 3648 g
E)1.00 mol, 32.0 g
A)0.455 mol, 7.28 g
B)0.455 mol, 14.6 g
C)228 mol, 7.14 g
D)228 mol, 3648 g
E)1.00 mol, 32.0 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate the number of moles in 95.0 g of O2, and the volume that it would occupy at 80.0°C and 3.50 atm.
A)11.9 mol, 266 L
B)5.94 mol, 11.1 L
C)5.94 mol, 49.2 L
D)2.97 mol, 24.6 L
E)2.97 mol, 5.57 L
A)11.9 mol, 266 L
B)5.94 mol, 11.1 L
C)5.94 mol, 49.2 L
D)2.97 mol, 24.6 L
E)2.97 mol, 5.57 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
57
Calculate the number of moles in 55 g of N2, and the volume that it would occupy at STP.
A)2.0 mol, 45 L
B)2.0 mol, 0.089 L
C)1.5 x 103 mol, 67 L
D)1.5 x 103 mol, 3.4 x 104 L
E)3.9 mol, 88 L
A)2.0 mol, 45 L
B)2.0 mol, 0.089 L
C)1.5 x 103 mol, 67 L
D)1.5 x 103 mol, 3.4 x 104 L
E)3.9 mol, 88 L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
58
The two balloons shown in the figure each have the same volume, temperature, and pressure. Which of the following statements regarding the two balloons is correct? 
A)The CO2 balloon has fewer molecules than the O2 balloon.
B)The density of the O2 balloon is greater than the density of the CO2 balloon.
C)The mass of the O2 balloon is greater than the mass of the CO2 balloon.
D)The CO2 balloon is more buoyant than the O2 balloon.
E)None of these statements is correct.

A)The CO2 balloon has fewer molecules than the O2 balloon.
B)The density of the O2 balloon is greater than the density of the CO2 balloon.
C)The mass of the O2 balloon is greater than the mass of the CO2 balloon.
D)The CO2 balloon is more buoyant than the O2 balloon.
E)None of these statements is correct.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
59
A balloon is filled with helium at sea level, and then taken into the mountains to an elevation of 7500 feet. Assuming the temperatures are the same, what will happen to the balloon?
A)The balloon will compress because the lower pressure will increase the speed of the molecules.
B)Nothing happens to the balloon because pressure has no affect on the behavior of gases.
C)The balloon expands because the molecules get bigger.
D)The balloon expands because the molecules move faster.
E)The balloon will expand because the external pressure in the mountains is lower.
A)The balloon will compress because the lower pressure will increase the speed of the molecules.
B)Nothing happens to the balloon because pressure has no affect on the behavior of gases.
C)The balloon expands because the molecules get bigger.
D)The balloon expands because the molecules move faster.
E)The balloon will expand because the external pressure in the mountains is lower.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
60
In January a balloon is taken inside where the temperature is 25°C. If the balloon is filled to 2.0 L at -20°C outside, what will happen to the balloon when taken inside?
A)The balloon expands because the higher temperature will increase the speed of the molecules.
B)Nothing happens to the balloon because temperature has no affect on the behavior of gases.
C)The balloon compresses because the higher temperature will cause the molecules get bigger.
D)The balloon expands because the higher temperature will cause the molecules to slow down.
E)The balloon compresses because the higher temperature will increase the speed of the molecules.
A)The balloon expands because the higher temperature will increase the speed of the molecules.
B)Nothing happens to the balloon because temperature has no affect on the behavior of gases.
C)The balloon compresses because the higher temperature will cause the molecules get bigger.
D)The balloon expands because the higher temperature will cause the molecules to slow down.
E)The balloon compresses because the higher temperature will increase the speed of the molecules.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
61
Two balloons are at the same temperature and pressure, and contain equal volumes of gas, but one is floating, and the other is not. Suggest a possible composition for the contents of the balloons.
A)The floating balloon contains CO2, while the other contains SO2.
B)The floating balloon contains SO2, while the other contains CO2.
C)The floating balloon contains Ne, while the other contains CO2.
D)The floating balloon contains Ar, while the other contains He.
E)None of these answers could be correct.
A)The floating balloon contains CO2, while the other contains SO2.
B)The floating balloon contains SO2, while the other contains CO2.
C)The floating balloon contains Ne, while the other contains CO2.
D)The floating balloon contains Ar, while the other contains He.
E)None of these answers could be correct.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
62
Calculate the density of NO2 in g/L at STP.
A)22.41 g/L
B)2.053 g/L
C)1.031 x 103 g/L
D)0.04462 g/L
E)0.4871 g/L
A)22.41 g/L
B)2.053 g/L
C)1.031 x 103 g/L
D)0.04462 g/L
E)0.4871 g/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
63
A sample of O2 is collected over water at 21°C. If the total pressure of the sample is 712 torr, what is the partial pressure of the O2? The vapor pressure of water at 21°C is 18.6 torr.
A)712 torr
B)731 torr
C)693 torr
D)38.3 torr
E)not enough information
A)712 torr
B)731 torr
C)693 torr
D)38.3 torr
E)not enough information

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
64
Calculate the number of moles and the mass of Ar gas present in a 3.00 L steel container if the pressure is 5.75 atm, and the temperature is 22.0°C.
A)1.00 mol, 40.0 g
B)0.712 mol, 28.4 g
C)9.56 mol, 382 g
D)6.20 x 104 mol, 1.55 x 103 g
E)4.59 x 103 mol, 1.84 x 105 g
A)1.00 mol, 40.0 g
B)0.712 mol, 28.4 g
C)9.56 mol, 382 g
D)6.20 x 104 mol, 1.55 x 103 g
E)4.59 x 103 mol, 1.84 x 105 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
65
The total pressure of a mixture of gases
A)is always constant.
B)increases as the temperature is lowered.
C)equals the sum of the partial pressures of the individual gases.
D)equals the difference between the partial pressures of the individual gases.
E)is proportional to the average of the boiling points of the gases.
A)is always constant.
B)increases as the temperature is lowered.
C)equals the sum of the partial pressures of the individual gases.
D)equals the difference between the partial pressures of the individual gases.
E)is proportional to the average of the boiling points of the gases.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
66
If a 4.00 L container is filled with N2 to a pressure of 895 torr at 53.0°C, calculate the mass of the nitrogen in the container.
A)0.176 g
B)4.93 g
C)134 g
D)5.68 g
E)0.202 g
A)0.176 g
B)4.93 g
C)134 g
D)5.68 g
E)0.202 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
67
A sample of CO2 is collected over water at 23°C. If the total pressure of the sample is 734 torr, what is the partial pressure of the CO2? The vapor pressure of water at 23°C is 21.2 torr.
A)734 torr
B)755 torr
C)713 torr
D)34.6 torr
E)not enough information
A)734 torr
B)755 torr
C)713 torr
D)34.6 torr
E)not enough information

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
68
Calculate the number of moles in 45.0 g of N2, and the pressure that it would exert at 20.0°C in a 5.00 L steel tank.
A)1.61 mol, 0.525 atm
B)1.61 mol, 7.74 atm
C)3.21 mol, 15.48 atm
D)3.21 mol, 1.05 atm
E)6.43 mol, 31.0 atm
A)1.61 mol, 0.525 atm
B)1.61 mol, 7.74 atm
C)3.21 mol, 15.48 atm
D)3.21 mol, 1.05 atm
E)6.43 mol, 31.0 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
69
Calculate the number of moles in 75.0 g of O2, and the pressure that it would exert at 80.0°C in a 5.00 L steel tank.
A)4.69 mol, 6.18 atm
B)4.69 mol, 27.2 atm
C)2.34 mol, 13.6 atm
D)2.34 mol, 3.09 atm
E)9.38 mol, 27.2 atm
A)4.69 mol, 6.18 atm
B)4.69 mol, 27.2 atm
C)2.34 mol, 13.6 atm
D)2.34 mol, 3.09 atm
E)9.38 mol, 27.2 atm

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
70
A sample of N2 is collected over water at 24°C. If the total pressure of the sample is 694 torr, what is the partial pressure of the N2? The vapor pressure of water at 24°C is 22.4 torr.
A)672 torr
B)694 torr
C)0.913 atm
D)716 torr
E)31.0 torr
A)672 torr
B)694 torr
C)0.913 atm
D)716 torr
E)31.0 torr

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following gases will have a density of 2.104 g/L at 303 K and 1.31 atm?
A)He
B)Ne
C)Ar
D)Kr
E)Xe
A)He
B)Ne
C)Ar
D)Kr
E)Xe

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
72
If a 7.00 L container is filled with O2 to a pressure of 995 torr at 33.0°C, calculate the mass of the oxygen in the container.
A)11.7 g
B)0.365 g
C)277 g
D)2.57 x 103 g
E)0.0854 g
A)11.7 g
B)0.365 g
C)277 g
D)2.57 x 103 g
E)0.0854 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
73
Two balloons are at the same temperature and pressure, and contain equal volumes of gas, but one is floating, and the other is not. The reason for this behavior is that:
A)the balloon that is not floating has the molecules more closely spaced than the other.
B)the balloon that is not floating has a gas with a higher molar mass than that of air.
C)the balloon that is floating has molecules with more kinetic energy.
D)the balloon that is not floating has molecules that have slowed down, since it was filled before the floating balloon.
E)the balloon that is floating has a more dense gas than the one that is not floating, so it holds up the balloon better.
A)the balloon that is not floating has the molecules more closely spaced than the other.
B)the balloon that is not floating has a gas with a higher molar mass than that of air.
C)the balloon that is floating has molecules with more kinetic energy.
D)the balloon that is not floating has molecules that have slowed down, since it was filled before the floating balloon.
E)the balloon that is floating has a more dense gas than the one that is not floating, so it holds up the balloon better.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
74
Calculate the density of Ne in g/L at 22.0°C and 735 torr.
A)22.41 g/L
B)8.22 x 103 g/L
C)6.12 x 102 g/L
D)0.806 g/L
E)1.24 g/L
A)22.41 g/L
B)8.22 x 103 g/L
C)6.12 x 102 g/L
D)0.806 g/L
E)1.24 g/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
75
Equal volumes of methane (CH4)and hydrogen (H2)gases under the same conditions of temperature and pressure have equal
A)numbers of molecules.
B)masses.
C)numbers of atoms.
D)numbers of covalent bonds.
E)average velocities.
A)numbers of molecules.
B)masses.
C)numbers of atoms.
D)numbers of covalent bonds.
E)average velocities.

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
76
Calculate the number of moles and the mass of He gas present in a 2.00 L steel container if the pressure is 6.50 atm, and the temperature is 42.0°C.
A)1.00 mol, 4.00 g
B)0.503 mol, 2.01 g
C)3.77 mol, 15.1 g
D)4.99 x 104 mol, 1.25 x 104 g
E)6.65 x 103 mol, 2.66 x 105 g
A)1.00 mol, 4.00 g
B)0.503 mol, 2.01 g
C)3.77 mol, 15.1 g
D)4.99 x 104 mol, 1.25 x 104 g
E)6.65 x 103 mol, 2.66 x 105 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
77
Calculate the density of Ar in g/L at 20.0°C and 695 torr.
A)22.41 g/L
B)1.71 x 104 g/L
C)1.18 x 103 g/L
D)1.52 g/L
E)8.86 g/L
A)22.41 g/L
B)1.71 x 104 g/L
C)1.18 x 103 g/L
D)1.52 g/L
E)8.86 g/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
78
If a 5.00 L container is filled with H2 to a pressure of 975 torr at 23.0°C, calculate the mass of the hydrogen in the container.
A)0.263 g
B)0.530 g
C)201 g
D)404 g
E)258 g
A)0.263 g
B)0.530 g
C)201 g
D)404 g
E)258 g

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
79
A sample of H2 is collected over water at 22°C. If the total pressure of the sample is 744 torr, what is the partial pressure of the H2? The vapor pressure of water at 22°C is 19.8 torr.
A)37.6 torr
B)0.979 atm
C)764 torr
D)724 torr
E)744 torr
A)37.6 torr
B)0.979 atm
C)764 torr
D)724 torr
E)744 torr

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck
80
Calculate the density of CO2 in g/L at STP
A)22.41 g/L
B)1.964 g/L
C)9.864 x 102 g/L
D)0.02272 g/L
E)0.5092 g/L
A)22.41 g/L
B)1.964 g/L
C)9.864 x 102 g/L
D)0.02272 g/L
E)0.5092 g/L

Unlock Deck
Unlock for access to all 121 flashcards in this deck.
Unlock Deck
k this deck


