Deck 11: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/119
Play
Full screen (f)
Deck 11: Solutions
1
Which of the following substances is a nonelectrolyte?
A)KMnO4
B)CH3CH2OH
C)NaOH
D)K2SO4
E)Na2H
A)KMnO4
B)CH3CH2OH
C)NaOH
D)K2SO4
E)Na2H
CH3CH2OH
2
Which of the following substances is a strong electrolyte?
A)CH3CH2OH
B)C8H18
C)LiOH
D)CO2
E)PCl5
A)CH3CH2OH
B)C8H18
C)LiOH
D)CO2
E)PCl5
LiOH
3
Which of the diagrams in the figure best represents an aqueous solution of Na2SO4? 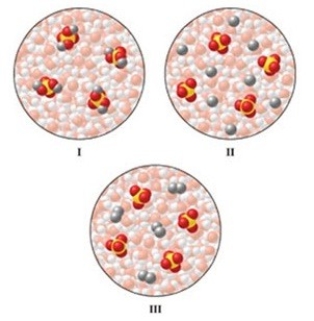
A)I
B)II
C)III
D)either I or III
E)none of these is correct

A)I
B)II
C)III
D)either I or III
E)none of these is correct
II
4
Which of the following equations describes what happens when CH3OH(l)dissolves in water?
A)CH3OH(l)→ C(aq)+ 4H(aq)+ O(aq)
B)CH3OH(l)→ C2−(aq)+ 4H+(aq)+ O2−(aq)
C)2CH3OH(l)→ 2C(aq)+ 4H2(aq)+ O2(aq)
D)CH3OH(l)→ CH3OH(aq)
E)CH3OH(l)+ H2O(l)→ CH5O2H(l)
A)CH3OH(l)→ C(aq)+ 4H(aq)+ O(aq)
B)CH3OH(l)→ C2−(aq)+ 4H+(aq)+ O2−(aq)
C)2CH3OH(l)→ 2C(aq)+ 4H2(aq)+ O2(aq)
D)CH3OH(l)→ CH3OH(aq)
E)CH3OH(l)+ H2O(l)→ CH5O2H(l)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
5
What ions, atoms, or molecules (in addition to the water molecules)are present after HNO3(l)mixes with water?
A)HNO3(l)
B)HNO3(aq)
C)H+(aq)+ NO3-(aq)
D)H(aq)+ NO3(aq)
E)H(aq)+ N(aq)+ 3O(aq)
A)HNO3(l)
B)HNO3(aq)
C)H+(aq)+ NO3-(aq)
D)H(aq)+ NO3(aq)
E)H(aq)+ N(aq)+ 3O(aq)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following should be most soluble in water?
A)Vitamin C (a very good hydrogen bonder)
B)Vitamin A (a mostly nonpolar molecule)
C)C6H6(l)
D)I2(s)
E)O2(g)
A)Vitamin C (a very good hydrogen bonder)
B)Vitamin A (a mostly nonpolar molecule)
C)C6H6(l)
D)I2(s)
E)O2(g)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements regarding the rule "like dissolves like" is incorrect?
A)Since cooking oil is composed primarily of hydrocarbons, it is insoluble in water.
B)Iodine, I2, is very soluble in water.
C)Ethanol, CH3CH2OH, is water-soluble.
D)Cooking oil, a nonpolar substance, is soluble in hexane, C6H14.
E)Potassium nitrate, KNO3, is soluble in water.
A)Since cooking oil is composed primarily of hydrocarbons, it is insoluble in water.
B)Iodine, I2, is very soluble in water.
C)Ethanol, CH3CH2OH, is water-soluble.
D)Cooking oil, a nonpolar substance, is soluble in hexane, C6H14.
E)Potassium nitrate, KNO3, is soluble in water.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following should be most soluble in water?
A)fat (a nonpolar triglyceride molecule)
B)C6H6(l)
C)CH3NH2(l)
D)I2(s)
E)O2(g)
A)fat (a nonpolar triglyceride molecule)
B)C6H6(l)
C)CH3NH2(l)
D)I2(s)
E)O2(g)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
9
What ions, atoms, or molecules (in addition to the water molecules)are present after Na2SO4(s)mixes with water?
A)Na2SO4(l)
B)Na2SO4(ag)
C)Na+(aq)+ NaSO4-(aq)
D)Na2SO32+(aq)+ O2-(aq)
E)2Na+(aq)+ SO42-(aq)
A)Na2SO4(l)
B)Na2SO4(ag)
C)Na+(aq)+ NaSO4-(aq)
D)Na2SO32+(aq)+ O2-(aq)
E)2Na+(aq)+ SO42-(aq)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following substances is a nonelectrolyte?
A)Na2SO4
B)CH3OH
C)KOH
D)LiNO3
E)RbCl
A)Na2SO4
B)CH3OH
C)KOH
D)LiNO3
E)RbCl

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following equations describes what happens when HCl(g)dissolves in water?
A)HCl(g)→ H+(aq)+ Cl−(aq)
B)HCl(g)→ H(aq)+ Cl(aq)
C)2HCl(g)→ H2(aq)+ Cl2(aq)
D)2HCl(g)→ H2(g)+ Cl2(g)
E)HCl(g)+ H2O(l)→ H3OCl(l)
A)HCl(g)→ H+(aq)+ Cl−(aq)
B)HCl(g)→ H(aq)+ Cl(aq)
C)2HCl(g)→ H2(aq)+ Cl2(aq)
D)2HCl(g)→ H2(g)+ Cl2(g)
E)HCl(g)+ H2O(l)→ H3OCl(l)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the diagrams in the figure best represents an aqueous solution of MgCl2? 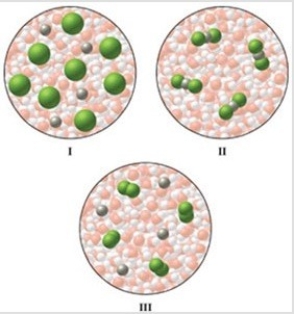
A)I
B)II
C)III
D)either I or III
E)none of these is correct

A)I
B)II
C)III
D)either I or III
E)none of these is correct

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following substances is a strong electrolyte?
A)CH3CH2OH
B)C6H6
C)KOH
D)SO2
E)PCl3
A)CH3CH2OH
B)C6H6
C)KOH
D)SO2
E)PCl3

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following substances is a nonelectrolyte?
A)NaNO3
B)LiOH
C)C6H12O6
D)LiCN
E)CsCl
A)NaNO3
B)LiOH
C)C6H12O6
D)LiCN
E)CsCl

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
15
What ions, atoms, or molecules are present after CH3CH2OH(I)mixes with water?
A)CH3CH2OH(aq)and H2O(l)
B)CH3CH2O-(aq), H+(aq), and H2O(l)
C)CH3CH2O-(aq), and H3O+(aq)
D)2C(aq), 6H(aq), and H2O2(l)
E)CH3+(aq), CH2O-(aq), H+(aq), and H2O(l)
A)CH3CH2OH(aq)and H2O(l)
B)CH3CH2O-(aq), H+(aq), and H2O(l)
C)CH3CH2O-(aq), and H3O+(aq)
D)2C(aq), 6H(aq), and H2O2(l)
E)CH3+(aq), CH2O-(aq), H+(aq), and H2O(l)

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following should be most soluble in hexane, C6H14?
A)NaCl
B)NaNO3
C)H2O
D)CH3OH
E)Br2
A)NaCl
B)NaNO3
C)H2O
D)CH3OH
E)Br2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following should be most soluble in benzene, C6H6?
A)H2O
B)CH3OH
C)I2
D)NaCl
E)NaNO3
A)H2O
B)CH3OH
C)I2
D)NaCl
E)NaNO3

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements regarding the rule "like dissolves like" is incorrect?
A)Since grease is nonpolar, it is not soluble in water.
B)Nitrogen, N2, is not very soluble in water.
C)Methanol, CH3OH, is not very water-soluble.
D)Butane, C4H10, is soluble in cooking oil, a nonpolar substance.
E)Calcium nitrate, Ca(NO3), is soluble in water.
A)Since grease is nonpolar, it is not soluble in water.
B)Nitrogen, N2, is not very soluble in water.
C)Methanol, CH3OH, is not very water-soluble.
D)Butane, C4H10, is soluble in cooking oil, a nonpolar substance.
E)Calcium nitrate, Ca(NO3), is soluble in water.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements regarding the rule "like dissolves like" is incorrect?
A)Since cooking oil is composed primarily of hydrocarbons, it is soluble in water.
B)Oxygen, O2, is not very soluble in water.
C)Methanol, CH3OH, is water-soluble.
D)Cooking oil, a nonpolar substance, is soluble in heptane, C7H16.
E)Sodium nitrate, NaNO3, is soluble in water.
A)Since cooking oil is composed primarily of hydrocarbons, it is soluble in water.
B)Oxygen, O2, is not very soluble in water.
C)Methanol, CH3OH, is water-soluble.
D)Cooking oil, a nonpolar substance, is soluble in heptane, C7H16.
E)Sodium nitrate, NaNO3, is soluble in water.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following substances is a strong electrolyte?
A)CH3OH
B)C6H14
C)C6H12O6
D)LiCl
E)SO2
A)CH3OH
B)C6H14
C)C6H12O6
D)LiCl
E)SO2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
21
What mass of sodium nitrate is dissolved in 455 g of a solution that is 15.0% by mass NaNO3?
A)68.3 g
B)30.3 g
C)4.40 × 102 g
D)3.03 × 103 g
E)15.0 g
A)68.3 g
B)30.3 g
C)4.40 × 102 g
D)3.03 × 103 g
E)15.0 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following statements regarding the solution process is incorrect?
A)When a solution is formed from an ionic compound, the anions and cations are evenly distributed throughout the solution.
B)When a polar covalent compound dissolves in water, dipole-dipole interactions take place between the water molecules and the solute molecules.
C)A nonpolar compound such as hexane, C6H14, forms London dispersion forces with a nonpolar solute, such as I2 during the solution process.
D)The solution process is always exothermic.
E)When an ionic solute dissolves, the entropy of the solution is usually greater than the original entropy of the crystalline form of the ionic substance.
A)When a solution is formed from an ionic compound, the anions and cations are evenly distributed throughout the solution.
B)When a polar covalent compound dissolves in water, dipole-dipole interactions take place between the water molecules and the solute molecules.
C)A nonpolar compound such as hexane, C6H14, forms London dispersion forces with a nonpolar solute, such as I2 during the solution process.
D)The solution process is always exothermic.
E)When an ionic solute dissolves, the entropy of the solution is usually greater than the original entropy of the crystalline form of the ionic substance.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
23
What mass of sodium chloride is dissolved in 365 g of a solution that is 14.0% by mass NaCl?
A)5110 g
B)26.1 g
C)351 g
D)51.1 g
E)14.0 g
A)5110 g
B)26.1 g
C)351 g
D)51.1 g
E)14.0 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
24
What is the percent-by-mass concentration of NaCl in a solution that is prepared by adding 14.0 g of NaCl to 75.0 g of water?
A)61.0%
B)15.7%
C)18.7%
D)24.0%
E)5.36%
A)61.0%
B)15.7%
C)18.7%
D)24.0%
E)5.36%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
25
When potassium nitrate is dissolved in water, the resulting solution feels cool to the touch. This means that
A)the strength of attraction between the solute particles is greater than that of the attraction between the solute and solvent.
B)the strength of attraction between the solute and solvent particles is greater than that of the attraction between the solute particles.
C)the strength of attraction between the solute particles is equal to that of the attraction between the solute and solvent.
D)hydrogen bonds must be broken, which is an exothermic process.
E)there is a decrease in entropy for the solution.
A)the strength of attraction between the solute particles is greater than that of the attraction between the solute and solvent.
B)the strength of attraction between the solute and solvent particles is greater than that of the attraction between the solute particles.
C)the strength of attraction between the solute particles is equal to that of the attraction between the solute and solvent.
D)hydrogen bonds must be broken, which is an exothermic process.
E)there is a decrease in entropy for the solution.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following increases the solubility of a gas in solution?
A)increasing gas pressure and increasing temperature
B)increasing gas pressure and decreasing temperature
C)decreasing gas pressure and increasing temperature
D)decreasing gas pressure and decreasing temperature
E)adding more water
A)increasing gas pressure and increasing temperature
B)increasing gas pressure and decreasing temperature
C)decreasing gas pressure and increasing temperature
D)decreasing gas pressure and decreasing temperature
E)adding more water

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
27
The solubility of potassium chloride is 34.2 g/100.0 g of water at 20ºC. Which of the following best describes a solution prepared by adding 58.3 g of potassium chloride to 150.0 g of water at this temperature?
A)34.2 g of KCl will dissolve and 24.1 g will remain undissolved.
B)51.3 g of KCl will dissolve and 7.0 g will remain undissolved.
C)24.1 g of KCl will dissolve and 34.2 g will remain undissolved.
D)58.3 g of KCl will dissolve and the solution will be unsaturated.
E)107 g of KCl will dissolve and the solution will be saturated.
A)34.2 g of KCl will dissolve and 24.1 g will remain undissolved.
B)51.3 g of KCl will dissolve and 7.0 g will remain undissolved.
C)24.1 g of KCl will dissolve and 34.2 g will remain undissolved.
D)58.3 g of KCl will dissolve and the solution will be unsaturated.
E)107 g of KCl will dissolve and the solution will be saturated.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
28
Many cold packs contain ammonium nitrate and water. The dissolving of ammonium nitrate is endothermic. Which of the following best describes the energy and entropy changes that occur for the NH4NO3 system when ammonium nitrate and water are mixed to make a solution?
A)There is a net energy increase and an entropy increase.
B)There is a net energy increase and an entropy decrease.
C)There is a net energy decrease and an entropy decrease.
D)There is no a net change in energy or entropy.
A)There is a net energy increase and an entropy increase.
B)There is a net energy increase and an entropy decrease.
C)There is a net energy decrease and an entropy decrease.
D)There is no a net change in energy or entropy.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following statements regarding solubility is incorrect?
A)A polar compound dissolves an ionic compound due to ion-dipole interactions.
B)A nonpolar solvent will not interact strongly enough with ions to dissolve an ionic compound.
C)Nonpolar liquids will dissolve in one another because the intermolecular forces in the pure liquids are weak, and there is an increase in entropy.
D)Most ionic solids are more soluble in water at higher temperatures than at lower temperatures.
E)Most gases are more soluble in water at higher temperatures than at lower temperatures.
A)A polar compound dissolves an ionic compound due to ion-dipole interactions.
B)A nonpolar solvent will not interact strongly enough with ions to dissolve an ionic compound.
C)Nonpolar liquids will dissolve in one another because the intermolecular forces in the pure liquids are weak, and there is an increase in entropy.
D)Most ionic solids are more soluble in water at higher temperatures than at lower temperatures.
E)Most gases are more soluble in water at higher temperatures than at lower temperatures.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following should be most soluble in ethanol, CH3CH2OH?
A)CH3NH2
B)CCl4
C)I2
D)C6H6
E)CO2
A)CH3NH2
B)CCl4
C)I2
D)C6H6
E)CO2

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
31
If the solubility of sodium chloride at 25ºC is 36.0 g/100 g water, which of the following best describes what eventually forms after 15.0 grams of sodium chloride is mixed with 50.0 grams of water at 25ºC?
A)a saturated solution with some undissolved solid
B)an unsaturated solution with some undissolved solid
C)a saturated solution with no undissolved solid
D)an unsaturated solution with no undissolved solid
E)a supersaturated solution
A)a saturated solution with some undissolved solid
B)an unsaturated solution with some undissolved solid
C)a saturated solution with no undissolved solid
D)an unsaturated solution with no undissolved solid
E)a supersaturated solution

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
32
Sodium chloride, NaCl, dissolves in water because
A)there are attractive forces between ions and water molecules.
B)there are no attractive forces between ions and water molecules.
C)the entropy of the solution is greater than the entropy of pure NaCl and pure H2O.
D)the entropy of the solution is less than the entropy of pure NaCl and pure H2O.
E)Both "there are attractive forces between ions and water molecules" and "the entropy of the solution is greater than the entropy of pure NaCl and pure H2O" are correct.
A)there are attractive forces between ions and water molecules.
B)there are no attractive forces between ions and water molecules.
C)the entropy of the solution is greater than the entropy of pure NaCl and pure H2O.
D)the entropy of the solution is less than the entropy of pure NaCl and pure H2O.
E)Both "there are attractive forces between ions and water molecules" and "the entropy of the solution is greater than the entropy of pure NaCl and pure H2O" are correct.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements regarding the solution process is incorrect?
A)In a sodium chloride solution, the ions interact with the water molecules through ion-dipole forces.
B)Hydration is the process whereby water molecules surround solute particles.
C)A hydrated cation is surrounded by the partially positive end of the water molecules.
D)When an ionic compound dissolves, the ionic bonds break.
E)Some of the hydrogen bonds among the water molecules must break.
A)In a sodium chloride solution, the ions interact with the water molecules through ion-dipole forces.
B)Hydration is the process whereby water molecules surround solute particles.
C)A hydrated cation is surrounded by the partially positive end of the water molecules.
D)When an ionic compound dissolves, the ionic bonds break.
E)Some of the hydrogen bonds among the water molecules must break.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following occurs when an ionic compound dissolves in water to form a solution?
A)Ionic bonds break.
B)Hydrogen bonds are disrupted.
C)H2O - Ion attractive forces form.
D)Entropy increases.
E)All of these
A)Ionic bonds break.
B)Hydrogen bonds are disrupted.
C)H2O - Ion attractive forces form.
D)Entropy increases.
E)All of these

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
35
What mass of sodium hydroxide is dissolved in 295 g of a solution that is 12.0% by mass NaOH?
A)3540 g
B)35.4 g
C)4.07 g
D)40.7 g
E)12.0 g
A)3540 g
B)35.4 g
C)4.07 g
D)40.7 g
E)12.0 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements regarding the solubility of oxygen in water is incorrect?
A)Water in contact with air contains a small amount of dissolved oxygen.
B)When water is heated, the first tiny bubbles that you see escaping from the warm water contain N2 and O2.
C)In warm weather, fish must come to the surface more often than in cool weather to get oxygen, since there is less dissolved oxygen in the water than in cooler weather.
D)If a sealed container has both oxygen and water, changing the pressure of the oxygen above the water will have no effect on the solubility of the oxygen in the water.
E)An increase in temperature causes an increase in the kinetic energy of the molecules in a solution, allowing dissolved oxygen molecules to escape the solution more readily.
A)Water in contact with air contains a small amount of dissolved oxygen.
B)When water is heated, the first tiny bubbles that you see escaping from the warm water contain N2 and O2.
C)In warm weather, fish must come to the surface more often than in cool weather to get oxygen, since there is less dissolved oxygen in the water than in cooler weather.
D)If a sealed container has both oxygen and water, changing the pressure of the oxygen above the water will have no effect on the solubility of the oxygen in the water.
E)An increase in temperature causes an increase in the kinetic energy of the molecules in a solution, allowing dissolved oxygen molecules to escape the solution more readily.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
37
What is the percent-by-mass concentration of KCl in a solution that is prepared by adding 13.0 g of KCl to 85.0 g of water?
A)15.3%
B)17.2%
C)13.3%
D)72.0%
E)65.4%
A)15.3%
B)17.2%
C)13.3%
D)72.0%
E)65.4%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
38
If the solubility of a solid substance is 18.2 g/100 g water, which of the following best describes what eventually forms after 10.0 grams of the substance is mixed with 50.0 grams of water?
A)a saturated solution with some undissolved solid
B)an unsaturated solution with some undissolved solid
C)a saturated solution with no undissolved solid
D)an unsaturated solution with no undissolved solid
E)a supersaturated solution
A)a saturated solution with some undissolved solid
B)an unsaturated solution with some undissolved solid
C)a saturated solution with no undissolved solid
D)an unsaturated solution with no undissolved solid
E)a supersaturated solution

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
39
Given that the solubility of potassium chlorate in water is 10.0 g/100 g water at 30°C, what mass of potassium chlorate is dissolved in 250 grams of a saturated solution at 30°C?
A)any mass greater than 25.0 g
B)any mass less than 10.0 g
C)any mass less than 25.0 g
D)25.0 g
E)10.0 g
A)any mass greater than 25.0 g
B)any mass less than 10.0 g
C)any mass less than 25.0 g
D)25.0 g
E)10.0 g

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
40
What is the percent-by-mass concentration of KOH in a solution that is prepared by adding 18.0 g of KOH to 95.0 g of water?
A)18.9%
B)15.9%
C)31.5%
D)5.28%
E)77.0%
A)18.9%
B)15.9%
C)31.5%
D)5.28%
E)77.0%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
41
A 200.0 g sample of rainwater contains 4.8 mg of sulfuric acid. How many parts per million of sulfuric acid are in the sample?
A)42 ppm
B)21 ppm
C)2.4 × 102 ppm
D)4.8 ppm
E)24 ppm
A)42 ppm
B)21 ppm
C)2.4 × 102 ppm
D)4.8 ppm
E)24 ppm

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
42
A solution contains 22.0 g of HCl dissolved in sufficient water to give a total mass of 125.0 g. What is the molality of the solution?
A)0.250 m
B)5.86 m
C)2.75 m
D)4.83 m
E)0.176 m
A)0.250 m
B)5.86 m
C)2.75 m
D)4.83 m
E)0.176 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
43
A solution of ethanol, C2H5OH, is prepared by dissolving 25.0 mL of ethanol in enough water to give a total volume of 250.0 mL. What is the percent-by-volume concentration of ethanol?
A)10.0%
B)9.09%
C)90.0%
D)1.00%
E)225%
A)10.0%
B)9.09%
C)90.0%
D)1.00%
E)225%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
44
A solution of acetic acid, CH3CO2H, is prepared by dissolving 30.0 mL of acetic acid in enough water to give a total volume of 150.0 mL. What is the percent-by-volume concentration of acetic acid?
A)16.7%
B)15.0%
C)30.0%
D)20.0%
E)25.0%
A)16.7%
B)15.0%
C)30.0%
D)20.0%
E)25.0%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
45
What is the percent-by-mass concentration of antifreeze (ethylene glycol, C2H6O2)in an aqueous solution that contains 420.0 g of ethylene glycol in 1.00 L of solution? The density of the solution is 1.05 g/mL.
A)2.50%
B)42.1%
C)40.0%
D)44.1%
E)60.0%
A)2.50%
B)42.1%
C)40.0%
D)44.1%
E)60.0%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
46
How many moles of NaCl are contained in 50.0 mL of 2.50 M NaCl?
A)2.50 moles
B)0.050 moles
C)5.00 moles
D)2.45 moles
E)0.125 moles
A)2.50 moles
B)0.050 moles
C)5.00 moles
D)2.45 moles
E)0.125 moles

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
47
What volume of 3.00 M NaOH contains 1.75 mol NaOH?
A)1.75 L
B)3.00 L
C)4.75 L
D)0.583 L
E)1.71 L
A)1.75 L
B)3.00 L
C)4.75 L
D)0.583 L
E)1.71 L

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
48
What is the percent-by-mass concentration of citric acid, C6H8O7, in an aqueous solution that contains 263.2 g of citric acid in 1.00 L of solution? The density of the solution is 1.10 g/mL.
A)73.7%
B)26.3%
C)23.9%
D)3.80%
E)29.0%
A)73.7%
B)26.3%
C)23.9%
D)3.80%
E)29.0%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
49
How many moles of NaOH are contained in 100.0 mL of 3.00 M NaOH?
A)0.300 moles
B)300.0 moles
C)33.3 moles
D)0.00300 moles
E)0.333 moles
A)0.300 moles
B)300.0 moles
C)33.3 moles
D)0.00300 moles
E)0.333 moles

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
50
A 150.0 g sample of ocean water contains 3.5 mg of mercury. How many parts per million of mercury are in the sample?
A)15 ppm
B)23 ppm
C)2.4 × 102 ppm
D)2.3 × 104 ppm
E)43 ppm
A)15 ppm
B)23 ppm
C)2.4 × 102 ppm
D)2.3 × 104 ppm
E)43 ppm

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
51
How many moles of HCl are contained in 75.0 mL of 2.00 M HCl?
A)2.00 moles
B)1.50 ×102 moles
C)0.150 moles
D)37.5 moles
E)26.7 moles
A)2.00 moles
B)1.50 ×102 moles
C)0.150 moles
D)37.5 moles
E)26.7 moles

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
52
What is the molal concentration of ions in a 2.40 m solution of Al(NO3)3?
A)2.40 m
B)7.20 m
C)9.60 m
D)0.800 m
E)31.2 m
A)2.40 m
B)7.20 m
C)9.60 m
D)0.800 m
E)31.2 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
53
What volume of 6.00 M NaOH contains 2.50 mol NaOH?
A)2.50 L
B)6.00 L
C)2.40 L
D)0.417 L
E)15.0 L
A)2.50 L
B)6.00 L
C)2.40 L
D)0.417 L
E)15.0 L

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
54
A 200.0 g sample of river water contains 6.4 mg of lead. How many parts per million of lead are in the sample?
A)6.5 ppm
B)21 ppm
C)3.2 × 102 ppm
D)32 ppm
E)54 ppm
A)6.5 ppm
B)21 ppm
C)3.2 × 102 ppm
D)32 ppm
E)54 ppm

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
55
What is the molal concentration of ions in a 1.60 m solution of Fe(NO3)3?
A)1.60 m
B)3.20 m
C)4.80 m
D)6.40 m
E)20.8 m
A)1.60 m
B)3.20 m
C)4.80 m
D)6.40 m
E)20.8 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
56
A solution of acetic acid, CH3CO2H, is prepared by dissolving 40.0 mL of acetic acid in enough water to give a total volume of 250.0 mL. What is the percent-by-volume concentration of acetic acid?
A)19.0%
B)16.0%
C)25.0%
D)40.0%
E)13.8%
A)19.0%
B)16.0%
C)25.0%
D)40.0%
E)13.8%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
57
What volume of 2.00 M HCl contains 0.50 mol HCl?
A)1.0 L
B)0.25 L
C)2.5 L
D)0.50 L
E)1.5 L
A)1.0 L
B)0.25 L
C)2.5 L
D)0.50 L
E)1.5 L

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
58
A solution contains 25.5 g of NaCl dissolved in sufficient water to give a total mass of 325.0 g. What is the molality of the solution?
A)0.146 m
B)0.785 m
C)1.34 m
D)1.46 m
E)134 m
A)0.146 m
B)0.785 m
C)1.34 m
D)1.46 m
E)134 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
59
What is the percent-by-mass concentration of hydrogen peroxide, H2O2, in an aqueous solution that contains 30.0 g of hydrogen peroxide in 1.00 L of solution? The density of the solution is 1.00 g/mL.
A)30.0%
B)3.00%
C)33.3%
D)31.0%
E)70.0%
A)30.0%
B)3.00%
C)33.3%
D)31.0%
E)70.0%

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
60
A solution contains 15.5 g of NaOH dissolved in sufficient water to give a total mass of 125.0 g. What is the molality of the solution?
A)3.54 m
B)0.124 m
C)0.00310 m
D)3.10 m
E)124 m
A)3.54 m
B)0.124 m
C)0.00310 m
D)3.10 m
E)124 m

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
61
What volume of 0.1452 M KOH is required to neutralize 25.00 mL of 0.1020 M H2SO4? (First, write a balanced equation for the reaction.)
A)17.79 mL
B)17.56 mL
C)8.781 mL
D)35.12 mL
E)71.18 mL
A)17.79 mL
B)17.56 mL
C)8.781 mL
D)35.12 mL
E)71.18 mL

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
62
When a 25.00 mL sample of H2SO4 is titrated with 0.2453 M NaOH, 32.47 mL of NaOH solution is required to neutralize the H2SO4. What is the molarity of the H2SO4?
A)0.3186 M
B)0.1889 M
C)0.3777 M
D)3.309 M
E)0.1593 M
A)0.3186 M
B)0.1889 M
C)0.3777 M
D)3.309 M
E)0.1593 M

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
63
Silver nitrate, AgNO3, can be used to test for the presence of chloride ions in solution, because it readily forms a precipitate of AgCl. What volume of 1.5 M AgNO3 will be required to react with 30.0 mL of a 0.45 M HCl solution AgNO3(aq)+ HCl(aq)→ AgCl(s)+ HNO3(aq)?
A)45 mL
B)9.0 mL
C)9.0 L
D)1.0 L
E)1.1 × 102 mL
A)45 mL
B)9.0 mL
C)9.0 L
D)1.0 L
E)1.1 × 102 mL

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
64
When a 25.00 mL sample of NaOH is titrated with 0.2523 M H2SO4, 26.69 mL of H2SO4 solution is required to neutralize the NaOH. What is the molarity of the NaOH?
A)0.1347 M
B)0.4726 M
C)0.5387 M
D)0.2694 M
E)0.2363 M
A)0.1347 M
B)0.4726 M
C)0.5387 M
D)0.2694 M
E)0.2363 M

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
65
How many moles of potassium iodide, KI, are required to precipitate all of the lead (II)ion from 25.0 mL of a 1.6 M Pb(NO3)2 solution? (First, write a balanced equation for the reaction.)
A)0.040 mole
B)0.080 mole
C)0.020 mole
D)64 mol
E)0.64 mol
A)0.040 mole
B)0.080 mole
C)0.020 mole
D)64 mol
E)0.64 mol

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
66
Calculate the freezing point of a 2.0 m solution of sucrose in water. Sucrose is a nonelectrolyte. The normal freezing point of pure water is 0.00ºC. Kf (water)= -1.86ºC/m.
A)-1.86ºC
B)-3.72ºC
C)-2.00ºC
D)3.72ºC
E)1.86ºC
A)-1.86ºC
B)-3.72ºC
C)-2.00ºC
D)3.72ºC
E)1.86ºC

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
67
How many moles of potassium iodide, KI, are required to precipitate all of the lead(II)ion from 50.0 mL of a 1.2 M Pb(NO3)2 solution? (First, write a balanced equation for the reaction.)
A)0.12 mole
B)0.060 mole
C)0.24 mole
D)0.030 mole
E)0.048 mole
A)0.12 mole
B)0.060 mole
C)0.24 mole
D)0.030 mole
E)0.048 mole

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
68
Calculate the boiling point of a 4.0 m solution of sucrose in water. Sucrose is a nonelectrolyte. The normal boiling point of pure water is 100.0ºC. Kb (water)= 0.52ºC/m.
A)2.1ºC
B)97.9ºC
C)96.0ºC
D)94.0ºC
E)102.1ºC
A)2.1ºC
B)97.9ºC
C)96.0ºC
D)94.0ºC
E)102.1ºC

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
69
Calculate the boiling point of a 4.0 m solution of KI in water. Remember that KI is an electrolyte. The normal boiling point of pure water is 100.0ºC. Kb (water)= 0.52ºC/m.
A)101.0 ºC
B)104.2ºC
C)96.0ºC
D)102.1ºC
E)4.16ºC
A)101.0 ºC
B)104.2ºC
C)96.0ºC
D)102.1ºC
E)4.16ºC

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
70
Silver nitrate, AgNO3, can be used to test for the presence of chloride ions in solution, because it readily forms a precipitate of AgCl. What volume of 2.0 M AgNO3 will be required to react with 50.0 mL of a 0.10 M HCl solution? AgNO3(aq)+ HCl(aq)→ AgCl(s)+ HNO3(aq)
A)0.25 mL
B)25 mL
C)5.0 × 101 mL
D)2.5 × 102 mL
E)2.5 mL
A)0.25 mL
B)25 mL
C)5.0 × 101 mL
D)2.5 × 102 mL
E)2.5 mL

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
71
What will happen to a blood cell that is placed in an aqueous solution that has a high salt concentration relative to the blood cell concentration?
A)The cell will shrink because there will be a net flow of water to the outside of the cell.
B)The cell will shrink because there will be a net flow of electrolytes to the outside of the cell.
C)The cell will expand because there will be a net flow of water to the inside of the cell.
D)The cell will expand because there will be a net flow of electrolytes to the inside of the cell.
E)Nothing will happen because the cell is impermeable.
A)The cell will shrink because there will be a net flow of water to the outside of the cell.
B)The cell will shrink because there will be a net flow of electrolytes to the outside of the cell.
C)The cell will expand because there will be a net flow of water to the inside of the cell.
D)The cell will expand because there will be a net flow of electrolytes to the inside of the cell.
E)Nothing will happen because the cell is impermeable.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
72
When a 25.00 mL sample of H2SO4 is titrated with 0.3423 M NaOH, 26.67 mL of NaOH solution is required to neutralize the H2SO4. What is the molarity of the H2SO4?
A)0.3652 M
B)0.3209 M
C)0.1604 M
D)0.1826 M
E)0.1988 M
A)0.3652 M
B)0.3209 M
C)0.1604 M
D)0.1826 M
E)0.1988 M

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
73
The EPA has determined that the maximum safe level of lead ion in drinking water is 15 ppb. If a sample of tap water has a lead ion concentration of 0.0065 ppm, what is the concentration of the lead ion in ppb, and is the water safe to drink? Assume the density of the solution is 1.00 g/mL.
A)65 ppb, not safe
B)6.5 ppb, safe
C)650 ppb, not safe
D)0.65 ppb, safe
E)6.5 × 10-6 ppm, safe
A)65 ppb, not safe
B)6.5 ppb, safe
C)650 ppb, not safe
D)0.65 ppb, safe
E)6.5 × 10-6 ppm, safe

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
74
Calculate the freezing point of a 1.0 m solution of Ca(NO3)2 in water. Remember that Mg(NO3)2 is an electrolyte. The normal freezing point of pure water is 0.00ºC. Kf (water)= -1.86ºC/m.
A)1.56 ºC
B)5.58ºC
C)1.86ºC
D)-5.58ºC
E)-1.86ºC
A)1.56 ºC
B)5.58ºC
C)1.86ºC
D)-5.58ºC
E)-1.86ºC

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
75
What will happen to a blood cell that is placed in pure water?
A)The cell will shrink because there will be a net flow of water to the outside of the cell.
B)The cell will shrink because there will be a net flow of electrolytes to the outside of the cell.
C)The cell will expand because there will be a net flow of water to the inside of the cell.
D)The cell will expand because there will be a net flow of electrolytes to the inside of the cell.
E)Nothing will happen because the cell is impermeable.
A)The cell will shrink because there will be a net flow of water to the outside of the cell.
B)The cell will shrink because there will be a net flow of electrolytes to the outside of the cell.
C)The cell will expand because there will be a net flow of water to the inside of the cell.
D)The cell will expand because there will be a net flow of electrolytes to the inside of the cell.
E)Nothing will happen because the cell is impermeable.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
76
Calculate the freezing point of a 2.0 m solution of NaCl in water. Remember that NaCl is an electrolyte. The normal freezing point of pure water is 0.00ºC. Kf (water)= -1.86ºC/m.
A)-2.08ºC
B)-3.72ºC
C)-7.44ºC
D)7.44ºC
E)3.74ºC
A)-2.08ºC
B)-3.72ºC
C)-7.44ºC
D)7.44ºC
E)3.74ºC

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
77
Consider two aqueous glucose solutions of different concentrations, separated by a semi-permeable membrane. Which of the following best describes the process of osmosis at the molecular level?
A)The smaller water molecules move in a net direction from the more dilute side to the more concentrated side.
B)The smaller water molecules move in a net direction from the more concentrated side to the more dilute side.
C)The larger glucose particles move in a net direction from the more dilute side to the more concentrated side.
D)The larger glucose particles move in a net direction from the more concentrated side to the more dilute side.
E)Osmosis will not occur.
A)The smaller water molecules move in a net direction from the more dilute side to the more concentrated side.
B)The smaller water molecules move in a net direction from the more concentrated side to the more dilute side.
C)The larger glucose particles move in a net direction from the more dilute side to the more concentrated side.
D)The larger glucose particles move in a net direction from the more concentrated side to the more dilute side.
E)Osmosis will not occur.

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
78
When a 25.00 mL sample of NaOH is titrated with 0.4523 M H2SO4, 36.69 mL of H2SO4 solution is required to neutralize the NaOH. What is the molarity of the NaOH?
A)0.3319 M
B)0.6638 M
C)1.328 M
D)0.6164 M
E)0.1988 M
A)0.3319 M
B)0.6638 M
C)1.328 M
D)0.6164 M
E)0.1988 M

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
79
The EPA has determined that the maximum safe level of lead ion in drinking water is 15 ppb. If a sample of tap water has a lead ion concentration of 0.016 ppm, what is the concentration of the lead ion in ppb, and is the water safe to drink? Assume the density of the solution is 1.00 g/mL.
A)160 ppb, not safe
B)16 ppb, not safe
C)1600 ppb, not safe
D)0.16 ppb, safe
E)1.6 × 10-6 ppm, safe
A)160 ppb, not safe
B)16 ppb, not safe
C)1600 ppb, not safe
D)0.16 ppb, safe
E)1.6 × 10-6 ppm, safe

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck
80
What volume of 0.1243 M KOH is required to neutralize 25.00 mL of 0.1540 M H2SO4? (First, write a balanced equation for the reaction.)
A)10.09 mL
B)30.97 mL
C)61.95 mL
D)20.18 mL
E)40.36 mL
A)10.09 mL
B)30.97 mL
C)61.95 mL
D)20.18 mL
E)40.36 mL

Unlock Deck
Unlock for access to all 119 flashcards in this deck.
Unlock Deck
k this deck


