Deck 19: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 19: Electrochemistry
1
Explain a redox reaction with an example.
In any electrochemical process, electrons flow from one chemical substance to another, driven by an oxidation-reduction (redox) reaction. A redox reaction occurs when electrons are transferred from a substance that is oxidized to one that is being reduced. The reductant is the substance that loses electrons and is oxidized in the process the oxidant is the species that gains electrons and is reduced in the process. A redox reaction can be described as two half-reactions, one representing the oxidation process and one the reduction process. For the reaction of zinc with bromine, the overall chemical reaction is as follows:
Zn(s) + Br2(aq) → Zn2+(aq) + 2Br−(aq)
The half-reactions are as follows:
Reduction half-reaction: Br2(aq) + 2e− → 2Br−(aq)
Oxidation half-reaction: Zn(s) → Zn2+(aq) + 2e−
Zn is the reductant in this reaction (it loses electrons), and Br2 is the oxidant (it gains electrons).
Zn(s) + Br2(aq) → Zn2+(aq) + 2Br−(aq)
The half-reactions are as follows:
Reduction half-reaction: Br2(aq) + 2e− → 2Br−(aq)
Oxidation half-reaction: Zn(s) → Zn2+(aq) + 2e−
Zn is the reductant in this reaction (it loses electrons), and Br2 is the oxidant (it gains electrons).
2
The anode is the site of the reduction half-reaction.
False
3
What is the purpose of a salt bridge in a galvanic cell?
A salt bridge is a U-shaped tube inserted into both solutions of a galvanic cell that contains a concentrated liquid or gelled electrolyte and completes the circuit between the anode and the cathode. The ions in the salt bridge are selected so that they do not interfere with the electrochemical reaction by being oxidized or reduced themselves or by forming a precipitate or complex; commonly used cations and anions are Na+ or K+ and NO3− or SO42−, respectively. The electrolyte in the salt bridge serves two purposes: it completes the circuit by carrying electrical charge and maintains electrical neutrality in both solutions by allowing ions to migrate between them. The identity of the salt in a salt bridge is unimportant, as long as the component ions do not react or undergo a redox reaction under the operating conditions of the cell. In the absence of a salt bridge or some other similar connection, the reaction would rapidly cease because electrical neutrality could not be maintained.
4
A galvanic cell differs from an electrolytic cell in that a galvanic cell _____.
A) consumes electrical energy from an external source
B) drives a nonspontaneous redox reaction
C) contains two electrodes to provide an external connection
D) uses the energy released during a spontaneous redox reaction to generate electricity
E) contains an electrolyte that connects the electrodes
A) consumes electrical energy from an external source
B) drives a nonspontaneous redox reaction
C) contains two electrodes to provide an external connection
D) uses the energy released during a spontaneous redox reaction to generate electricity
E) contains an electrolyte that connects the electrodes

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
A voltaic cell consumes electrical energy from an external source.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
The oxidant is the species that loses electrons in the process.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
A galvanic cell consists of two beakers. One beaker contains a strip of lead immersed in aqueous sulfuric acid, and the other contains a platinum electrode immersed in aqueous nitric acid. The two solutions are connected by a salt bridge, and the electrodes are connected by a wire. Current begins to flow, and bubbles of a gas appear at the platinum electrode. The spontaneous redox reaction that occurs is described by the following balanced chemical equation: Which of the following is the oxidation half-reaction that occurs in the anode?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called a(n) _____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
Measured redox potentials are independent of the temperature of the system.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
In any redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
A galvanic cell consists of two beakers. One beaker contains a strip of lead immersed in aqueous sulfuric acid, and the other contains a platinum electrode immersed in aqueous nitric acid. The two solutions are connected by a salt bridge, and the electrodes are connected by a wire. Current begins to flow, and bubbles of a gas appear at the platinum electrode. The spontaneous redox reaction that occurs is described by the following balanced chemical equation: Which of the following is the cell diagram for the galvanic cell?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
A galvanic cell consists of two beakers. One beaker contains a strip of lead immersed in aqueous sulfuric acid, and the other contains a platinum electrode immersed in aqueous nitric acid. The two solutions are connected by a salt bridge, and the electrodes are connected by a wire. Current begins to flow, and bubbles of a gas appear at the platinum electrode. The spontaneous redox reaction that occurs is described by the following balanced chemical equation: Which of the following is the reduction half-reaction that occurs in the cathode?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
_____ is the study of the relationship between electricity and chemical reactions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
A(n) _____ is an ionic substance or solution that allows ions to transfer between the electrode compartments, thereby maintaining the system's electrical neutrality.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
The standard cell potential is the sum of the tabulated reduction potentials of the two half-reactions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
Compare and contrast galvanic cells and electrolytic cells.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
The _____ of the cell is the difference in electrical potential between two half-reactions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
The identity of the salt in a salt bridge is unimportant, as long as the component ions do not react or undergo a redox reaction under the operating conditions of the cell.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
A(n) _____ is a U-shaped tube inserted into both solutions of a galvanic cell that contains a concentrated liquid or gelled electrolyte and completes the circuit between the anode and the cathode.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
_____ are the reactions that represent either the oxidation half or the reduction half of a redox reaction.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
The cell potential values are independent of the stoichiometric coefficients for the half-reaction.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
If the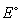 of the zinc anode is -0.76 V and the
of the zinc anode is -0.76 V and the 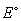 of the copper cathode is 0.28 V, what is the
of the copper cathode is 0.28 V, what is the 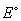 of the galvanic cell?
of the galvanic cell?
A) 0.480 V
B) 0.760 V
C) -1.04 V
D) -0.480 V
E) 1.04 V
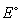 of the zinc anode is -0.76 V and the
of the zinc anode is -0.76 V and the 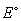 of the copper cathode is 0.28 V, what is the
of the copper cathode is 0.28 V, what is the 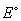 of the galvanic cell?
of the galvanic cell?A) 0.480 V
B) 0.760 V
C) -1.04 V
D) -0.480 V
E) 1.04 V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
The physical states of the reactants and the products in a half-reaction must be identical to those in the overall reaction.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
The _____ is universally used as a reference electrode and is assigned a standard potential of 0V.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
Explain the process of measuring standard electrode potentials.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following elements is most likely to be the strongest oxidant?
A) Chlorine
B) Hydrogen peroxide
C) Fluorine
D) Lead
E) Oxygen
A) Chlorine
B) Hydrogen peroxide
C) Fluorine
D) Lead
E) Oxygen

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
Explain the use of reference electrodes with examples.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following species can oxidize MnO2(s) to under standard conditions?
A) Sn4+
B) Sn2+
C) Al3+
D) Li+
E) Fe3+
A) Sn4+
B) Sn2+
C) Al3+
D) Li+
E) Fe3+

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following elements is most likely to be the strongest reductant in aqueous solution?
A) Li+
B) Al3+
C) Zn2+
D) Sn4+
E) Cu2+
A) Li+
B) Al3+
C) Zn2+
D) Sn4+
E) Cu2+

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
_____ electrodes are designed so that their potential depends on only the concentration of the desired species.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
All reactants that lie above the standard hydrogen electrode in the table are stronger reductants than H+.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
For the reaction , what is the final balanced equation using half-reactions?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
_____ is defined as the potential of a cell measured under standard conditions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
When using a galvanic cell to measure the concentration of a substance, the potential of the _____ is related to the concentration of the substance being measured.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
The reference electrode should be physically isolated from the solution of interest.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
The potential of a half-reaction measured against the sunder standard conditions is called the _____ for that half-reaction.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
If the of the galvanic cell is 0.98 V and the of the copper anode is -0.34 V, what is the of the hydrogen cathode?
A) 1.32 V
B) 0.34 V.
C) -0.64 V
D) 0.64 V
E) 0.34 V
A) 1.32 V
B) 0.34 V.
C) -0.64 V
D) 0.64 V
E) 0.34 V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
If the of the galvanic cell is 1.24 V and the of the cathode is 0.57, what is the of the anode?
A) -0.67 V
B) -0.57 V
C) 1.81 V
D) -1.81 V
E) 0.67 V
A) -0.67 V
B) -0.57 V
C) 1.81 V
D) -1.81 V
E) 0.67 V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following species can reduce PbSO4 to Pb under standard conditions?
A) Sn2+
B) Fe2+
C) Cu2+
D) Ni2+
E) I2
A) Sn2+
B) Fe2+
C) Cu2+
D) Ni2+
E) I2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
Any species on the left side of a half-reaction will spontaneously oxidize any species on the right side of another half-reaction that lies above it in the table.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
Derive the relationship between cell potential and the equilibrium constant?

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
If work is being done by a system on its surroundings, it is expressed as a positive number.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
If the overall cell potential is equal to 1.56 V and two electrons are transferred in the overall reaction, what is the equilibrium constant for the reaction? Given: F = .
A) 1.54×1053
B) 2.78×1052
C) 3.87×1053
D) 5.74×1052
E) 6.98×1053
A) 1.54×1053
B) 2.78×1052
C) 3.87×1053
D) 5.74×1052
E) 6.98×1053

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
If the equilibrium constant for the reaction is equal to 4.70 ×1070 and three electrons are transferred in the overall reaction, what is the overall cell potential? Given: F = .
A) 1.39 V
B) -2.84 V
C) 2.56 V
D) -1.25 V
E) 0.76 V
A) 1.39 V
B) -2.84 V
C) 2.56 V
D) -1.25 V
E) 0.76 V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
If the reaction 2Ce4+(aq) + 2Cl?(aq) ? 2Ce3+(aq) + Cl2(g) is likely to occur spontaneously under normal conditions, what is the for the reaction?(Use data in the Table 19.2)
A) -0.36V
B) 1.72 V
C) 1.36 V
D) 3.08 V
E) 0.36 V
A) -0.36V
B) 1.72 V
C) 1.36 V
D) 3.08 V
E) 0.36 V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
_____ is the fundamental SI unit of electric current.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
If = - 0.76 V, what is the value of the solubility product of the salt?
A) 2.7×10-12
B) 1.4×10-13
C) 3.9×10-13
D) 5.6×10-12
E) 7.2×10-13
A) 2.7×10-12
B) 1.4×10-13
C) 3.9×10-13
D) 5.6×10-12
E) 7.2×10-13

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
The potentials of two reductive half-reactions can be added to obtain the potential of a third reductive half-reaction.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
The amount of energy available to do electrical work depends on the total number of electrons that are transferred from the reductant to the oxidant during the course of a reaction.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
If the cell potential is equal to 0.240 V and the total charge transferred during the reaction is equal to 1.93×105
, what is the maximum work produced by the electrochemical cell?
A) 67.2 kJ/mol
B) -46.3 kJ/mol
C) 27.8 kJ/mol
D) 80.5 kJ/mol
E) -12.9 kJ/mol
, what is the maximum work produced by the electrochemical cell?
A) 67.2 kJ/mol
B) -46.3 kJ/mol
C) 27.8 kJ/mol
D) 80.5 kJ/mol
E) -12.9 kJ/mol

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
If the value of Q = 2.5×1032 and three electrons are transferred in the overall reaction, what is the value of ? Given: Ecell = 0.
A) 0.63 V
B) -0.71 V
C) -0.38 V
D) 0.28 V
E) 0.45 V
A) 0.63 V
B) -0.71 V
C) -0.38 V
D) 0.28 V
E) 0.45 V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
_____ is the SI unit of measure for the number of electrons that pass a given point in one second.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
If the free-energy change for the reaction is -87.5 kJ and the cell potential is equal to 0.18 V, how many moles of electrons are used in the reaction? Given: F = .
A) Two
B) Three
C) Four
D) Five
E) Six
A) Two
B) Three
C) Four
D) Five
E) Six

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
If = 0.58 V and two electrons are transferred in the overall reaction, what is the value of Q? Given: Ecell = 0.
A) 2.5×1018
B) 1.9×1019
C) 5.4×1018
D) 7.2×1019
E) 4.2×1019
A) 2.5×1018
B) 1.9×1019
C) 5.4×1018
D) 7.2×1019
E) 4.2×1019

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
If = 1.60 V and = 1.25 V, what is the free energy change for the reaction? Given: Total charge transferred during the reaction is equal to 1.93×105 .
A) -74.0 kJ/mol
B) -12.3 kJ/mol
C) -67.6 kJ/mol
D) -28.4 kJ/mol
E) -50.1 kJ/mol
A) -74.0 kJ/mol
B) -12.3 kJ/mol
C) -67.6 kJ/mol
D) -28.4 kJ/mol
E) -50.1 kJ/mol

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
Explain the process of determining the oxidants and reductants based on the standard potentials of half-reactions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
If the cell potential is equal to 0.760 V and the total charge transferred during the reaction is equal to 2.89×105
, what is the change in free-energy for the reaction?
A) 3.88 *102 kJ/mol
B) -1.32 * 102 kJ/mol
C) -4.29 *102 kJ/mol
D) 6.16 * 102 kJ/mol
E) -2.20 * 102 kJ/mol
, what is the change in free-energy for the reaction?
A) 3.88 *102 kJ/mol
B) -1.32 * 102 kJ/mol
C) -4.29 *102 kJ/mol
D) 6.16 * 102 kJ/mol
E) -2.20 * 102 kJ/mol

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
Multiplying the charge on the electron by Avogadro's number gives us the charge on 1 mol of electrons, which is called the _____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
A galvanic cell is constructed with a standard Fe/Fe2+ couple in one compartment and a modified hydrogen electrode in the second compartment. The pressure of hydrogen gas is 1.0 atm, but [H+] in the second compartment is unknown. What is the pH of the solution in the second compartment if the measured potential in the cell is 0.17 V at ?
A) 3.4
B) 1.9
C) 6.8
D) 8.2
E) 4.7
A) 3.4
B) 1.9
C) 6.8
D) 8.2
E) 4.7

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
For the reaction , = -0.59 V. What is the E for the reaction? Given: [Fe3+] = 0.017 M, [Fe2+] = 0.50 M, [Cl-] = 0.035 M, = 1 atm, and T = 298 K.
A) 0.34 V
B) -0.76 V
C) -0.28 V
D) 0.57 V
E) 0.82 V
A) 0.34 V
B) -0.76 V
C) -0.28 V
D) 0.57 V
E) 0.82 V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
Explain the Leclanche dry cell and state its half-reactions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
In a lithium-iodine battery, the anode is a solid complex of I2 and the cathode is lithium metal.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
Explain fuel cells with an example. State its half-reactions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
The alkaline battery has a longer shelf life and more constant output voltage than the Leclanche dry cell.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is the reaction that occurs at the cathode of a button battery?
A) 2MnO2(s) + 2NH4+(aq) + 2e? ? Mn2O3(s) + 2NH3(aq) + H2O(l)
B) I2(s) + 2e? ? 2I?(LiI)
C) 2NiO(OH)(s) + 2H2O(l) + 2e? ? 2Ni(OH)2(s) + 2OH?(aq)
D) PbO2(s)+ (aq)+3H+(aq)+2e - ?PbSO4(s)+2H2O(l)
E) HgO(s) + H2O(l) + 2e? ? Hg(l) + 2OH?(aq)
A) 2MnO2(s) + 2NH4+(aq) + 2e? ? Mn2O3(s) + 2NH3(aq) + H2O(l)
B) I2(s) + 2e? ? 2I?(LiI)
C) 2NiO(OH)(s) + 2H2O(l) + 2e? ? 2Ni(OH)2(s) + 2OH?(aq)
D) PbO2(s)+ (aq)+3H+(aq)+2e - ?PbSO4(s)+2H2O(l)
E) HgO(s) + H2O(l) + 2e? ? Hg(l) + 2OH?(aq)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
The _____ is a battery that consists of a Leclanché cell adapted to operate under alkaline conditions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
The anode of each cell in a lead storage battery is a grid of spongy lead metal, and the cathode is a similar grid containing powdered lead dioxide.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
The NiMH battery is more advantageous than the NiCad battery in that _____.
A) it has a lower self-discharge rate
B) it is insensitive to recharging memory
C) it has an unlimited service life
D) it requires lower maintenance
E) it is less expensive
A) it has a lower self-discharge rate
B) it is insensitive to recharging memory
C) it has an unlimited service life
D) it requires lower maintenance
E) it is less expensive

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
An electrochemical cell in which the anode and cathode compartments are identical except for the concentration of a reactant is called a(n) _____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
During cathodic protection, a more reactive metal such as zinc becomes the cathode and iron becomes the anode.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is the half-reaction that occurs at the cathode of the Leclanche dry cell?
A) PbO2 (s)+ (aq)+3H+(aq)+2e- ?PbSO4(s)+2H2O(l)
B) 2NiO(OH)(s) + 2H2O(l) + 2e? ? 2Ni(OH)2(s) + 2OH?(aq)
C) HgO(s) + H2O(l) + 2e? ? Hg(l) + 2OH?(aq)
D) 2MnO2(s) + 2NH4+(aq) + 2e? ? Mn2O3(s) + 2NH3(aq) + H2O(l)
E) Ag2O(s) + H2O(l) + 2e? ? 2Ag(s) + 2OH?(aq)
A) PbO2 (s)+ (aq)+3H+(aq)+2e- ?PbSO4(s)+2H2O(l)
B) 2NiO(OH)(s) + 2H2O(l) + 2e? ? 2Ni(OH)2(s) + 2OH?(aq)
C) HgO(s) + H2O(l) + 2e? ? Hg(l) + 2OH?(aq)
D) 2MnO2(s) + 2NH4+(aq) + 2e? ? Mn2O3(s) + 2NH3(aq) + H2O(l)
E) Ag2O(s) + H2O(l) + 2e? ? 2Ag(s) + 2OH?(aq)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
Which battery is used to provide the starting power in virtually every automobile and marine engine?
A) Lead-acid battery
B) Nickel-cadmium battery
C) Lithium-iodine battery
D) Leclanche dry cell
E) Alkaline battery
A) Lead-acid battery
B) Nickel-cadmium battery
C) Lithium-iodine battery
D) Leclanche dry cell
E) Alkaline battery

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following reactions occurs at the cathode during the formation of rust?
A)
B)
C) Zn(s) ? Zn2+(aq) + 2e?
D) 2H2(g) ? 4H+ + 4e?
E)
A)
B)
C) Zn(s) ? Zn2+(aq) + 2e?
D) 2H2(g) ? 4H+ + 4e?
E)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following is the overall reaction of the Leclanche dry cell?
A) Zn(s) + 2HgO(s)→Hg(l)+ZnO(s)
B) 2MnO2(s) + 2NH4Cl(aq) + Zn(s) → Mn2O3(s) + Zn(NH3)2Cl2(s) + H2O(l)
C) Zn(s) + 2Ag2O(s)→2Ag(s)+ZnO(s)
D) Cd(s)+2NiO(OH)(s)+2H2O(l)→Cd(OH)2(s)+2Ni(OH)2(s)
E) Pb(s) + PbO2(s)+2HSO4−(aq)+2H+(aq)→2PbSO4(s)+2H2O(l)
A) Zn(s) + 2HgO(s)→Hg(l)+ZnO(s)
B) 2MnO2(s) + 2NH4Cl(aq) + Zn(s) → Mn2O3(s) + Zn(NH3)2Cl2(s) + H2O(l)
C) Zn(s) + 2Ag2O(s)→2Ag(s)+ZnO(s)
D) Cd(s)+2NiO(OH)(s)+2H2O(l)→Cd(OH)2(s)+2Ni(OH)2(s)
E) Pb(s) + PbO2(s)+2HSO4−(aq)+2H+(aq)→2PbSO4(s)+2H2O(l)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
A battery is a galvanic cell that requires a constant external supply of one or more reactants to generate electricity.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following metals is most likely to protect iron from corrosion?
A) Sn2+
B) Ni2+
C) Cr3+
D) Zn2+
E) Cu2+
A) Sn2+
B) Ni2+
C) Cr3+
D) Zn2+
E) Cu2+

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is the overall reaction for the formation of rust?
A) 2MnO2(s) + 2NH4Cl(aq) + Zn(s) → Mn2O3(s) + Zn(NH3)2Cl2(s) + H2O(l)
B) Cd(s)+2NiO(OH)(s)+2H2O(l)→Cd(OH)2(s)+2Ni(OH)2(s)
C) 4Fe2+(aq) + O2(g) + (2 + 4x)H2O → 2Fe2O3·xH2O + 4H+(aq)
D) Zn(s) + 2MnO2(s)→ZnO(s)+Mn2O3(s)
E) 2Zn(s) + O2(g)+4H+(aq)→2Zn2+(aq)+2H2O(l)
A) 2MnO2(s) + 2NH4Cl(aq) + Zn(s) → Mn2O3(s) + Zn(NH3)2Cl2(s) + H2O(l)
B) Cd(s)+2NiO(OH)(s)+2H2O(l)→Cd(OH)2(s)+2Ni(OH)2(s)
C) 4Fe2+(aq) + O2(g) + (2 + 4x)H2O → 2Fe2O3·xH2O + 4H+(aq)
D) Zn(s) + 2MnO2(s)→ZnO(s)+Mn2O3(s)
E) 2Zn(s) + O2(g)+4H+(aq)→2Zn2+(aq)+2H2O(l)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
Lithium-iodine batteries are disposable and cannot be recharged once they are discharged.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
The _____ is a battery consisting of an electrolyte that is an acidic water-based paste containing MnO2, NH4Cl, ZnCl2, graphite, and starch.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is the overall reaction of a fuel cell?
A) Pb(s) + PbO2(s)+2HSO4- (aq)+2H+(aq)→2PbSO4(s)+2H2O(l)
B) Cd(s)+2NiO(OH)(s)+2H2O(l)→Cd(OH)2(s)+2Ni(OH)2(s)
C) 2H2(g)+O2(g)→2H2O(g)
D) 2Li(s) + I2(s)→2LiI(s)
E) 2MnO2(s) + 2NH4Cl(aq) + Zn(s) → Mn2O3(s) + Zn(NH3)2Cl2(s) + H2O(l)
A) Pb(s) + PbO2(s)+2HSO4- (aq)+2H+(aq)→2PbSO4(s)+2H2O(l)
B) Cd(s)+2NiO(OH)(s)+2H2O(l)→Cd(OH)2(s)+2Ni(OH)2(s)
C) 2H2(g)+O2(g)→2H2O(g)
D) 2Li(s) + I2(s)→2LiI(s)
E) 2MnO2(s) + 2NH4Cl(aq) + Zn(s) → Mn2O3(s) + Zn(NH3)2Cl2(s) + H2O(l)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck


