Deck 6: The Structure of Atoms
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/110
Play
Full screen (f)
Deck 6: The Structure of Atoms
1
According to Max Planck, at low temperatures, radiation with only relatively low frequencies is emitted, corresponding to low-energy quanta.
True
2
What is wavelength?
A) The number of horizontal troughs in a wave.
B) The number of vertical peaks in a wave.
C) The number of oscillations in a wave.
D) The distance between two points in a wave.
E) The distance travelled by a wave per unit time.
A) The number of horizontal troughs in a wave.
B) The number of vertical peaks in a wave.
C) The number of oscillations in a wave.
D) The distance between two points in a wave.
E) The distance travelled by a wave per unit time.
The distance between two points in a wave.
3
Visible light has wavelengths between _____.
700 nm to 400nm
4
Which of the following has the largest wavelength?
A) Gamma rays
B) Infrared rays
C) Ultraviolet rays
D) Microwaves
E) Radiowaves
A) Gamma rays
B) Infrared rays
C) Ultraviolet rays
D) Microwaves
E) Radiowaves

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
5
Electromagnetic radiation is transmitted through space by the periodic oscillations of _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
6
A laser pointer emits a deep red beam with a wavelength of 670 nm. What is the frequency of the deep red beam?
A) 894 Hz
B) 8.94 × 1014 Hz
C) 448 Hz
D) 4.48 × 1014 Hz
E) 6.70 × 10-9 Hz
A) 894 Hz
B) 8.94 × 1014 Hz
C) 448 Hz
D) 4.48 × 1014 Hz
E) 6.70 × 10-9 Hz

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
7
The wavelength of radiation emitted by a substance is dependant on its composition.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
8
Gamma rays have the greatest energy and wavelength among all electromagnetic radiation.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
9
All electromagnetic radiation is propagated with the same _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
10
The frequency of a wave is directly proportional to the speed of the wave.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
11
The wavelength of a wave is the distance between a wave crest and the adjacent trough.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
12
Blackbody radiation is the radiation emitted by a body when it is heated.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
13
_____ is the SI unit for frequency which can also be represented as s-1.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
14
If a wave has a wavelength of 3256 m and a frequency of 9.180 × 104 s−1, its velocity is _____ m/s.
A) 354.68
B) 3256
C) 2.998 × 108
D) 29980
E) 3.540 × 108
A) 354.68
B) 3256
C) 2.998 × 108
D) 29980
E) 3.540 × 108

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following has the highest energy?
A) Radio waves
B) Infrared rays
C) Visible light
D) Ultraviolet rays
E) X-rays
A) Radio waves
B) Infrared rays
C) Visible light
D) Ultraviolet rays
E) X-rays

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
16
A wave is a periodic oscillation that transmits _____ through space.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
17
A wave belonging to the electromagnetic spectrum has a frequency of 440.0 MHz. What is its wavelength?
A) 0.6818 m
B) 2.880 m
C) 1.004 m
D) 0.006000 m
E) 1.442 m
A) 0.6818 m
B) 2.880 m
C) 1.004 m
D) 0.006000 m
E) 1.442 m

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
18
Electromagnetic waves can only be transmitted in the Earth's atmosphere.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
19
Write a note on the electromagnetic radiation and the electromagnetic spectrum.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
20
All forms of electromagnetic radiation have the same speed of transmission.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
21
The unit of the Planck's constant is _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
22
The ground state of an electron is associated with the lowest energy.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
23
What is the smallest possible unit of energy called?
A) Photon
B) Erg
C) Quark
D) Positron
E) Quantum
A) Photon
B) Erg
C) Quark
D) Positron
E) Quantum

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
24
A photon is a quantum of radiant energy possessing a particular energy.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
25
When a pure sample of an individual element is heated (as in a discharge tube), a continuous spectrum of electromagnetic radiation is emitted by the sample.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is true of the photoelectric effect?
A) The number of electrons emitted by a metallic surface is independent of the frequency of incident light.
B) The number of electrons emitted by a metallic surface is independent of the intensity of light at any frequency.
C) The number of electrons emitted by a metallic surface is directly proportional to the intensity of incident light above the threshold frequency.
D) The number of electrons emitted by a metallic surface is proportional to the frequency of incident light only below a certain light intensity.
E) The number of electrons emitted by a metallic surface is inversely proportional to the intensity and frequency of incident light.
A) The number of electrons emitted by a metallic surface is independent of the frequency of incident light.
B) The number of electrons emitted by a metallic surface is independent of the intensity of light at any frequency.
C) The number of electrons emitted by a metallic surface is directly proportional to the intensity of incident light above the threshold frequency.
D) The number of electrons emitted by a metallic surface is proportional to the frequency of incident light only below a certain light intensity.
E) The number of electrons emitted by a metallic surface is inversely proportional to the intensity and frequency of incident light.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
27
Explain Einstein's hypothesis to explain the photo electric effect.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
28
A crystal emits a light of wavelength 720.0 nm. What is the light's energy in joules of a mole photons
A) 4.164 × 1010 J/mol
B) 6.625 × 10 -34 J/mol
C) 1.661 × 105 J/mol
D) 1.507 × 1015 J/mol
E) 41.120 × 1014 J/mol
A) 4.164 × 1010 J/mol
B) 6.625 × 10 -34 J/mol
C) 1.661 × 105 J/mol
D) 1.507 × 1015 J/mol
E) 41.120 × 1014 J/mol

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
29
Above the threshold frequency of a metal, the amount of electrons emitted by the metal when light is incident on it is proportional to the _____ of the light.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
30
The Pfund series of lines are due to transitions from higher-energy orbits to orbits with n = 5.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
31
To explain the line emission spectra of hydrogen in the visible region, Balmer proposed the following formula 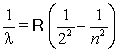 . According to this formula for what value of n would the energy of the emitted wavelength be the greatest?
. According to this formula for what value of n would the energy of the emitted wavelength be the greatest?
A) 3
B) 4
C) 5
D) 6
E) 7
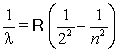 . According to this formula for what value of n would the energy of the emitted wavelength be the greatest?
. According to this formula for what value of n would the energy of the emitted wavelength be the greatest?A) 3
B) 4
C) 5
D) 6
E) 7

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
32
What is the energy of a single photon emitted by a laser emitting light at 540.0 nm?
A) 3.679 × 10 -19 J
B) 6.625 × 10 -34 J
C) 4.312 × 10 -34 J
D) 1.806 × 10 -19 J
E) 5.418× 10 -19 J
A) 3.679 × 10 -19 J
B) 6.625 × 10 -34 J
C) 4.312 × 10 -34 J
D) 1.806 × 10 -19 J
E) 5.418× 10 -19 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
33
In the emission spectrum of hydrogen the Brackett series of lines corresponds to transition of electrons from the excited states to the n1=4 orbit. What is the wavelength of the energy line n2=9 in the Brackett series?
A) 1350 nm
B) 5.50 × 105 nm
C) 1.09 nm
D) 1820 nm
E) 0.0123 m
A) 1350 nm
B) 5.50 × 105 nm
C) 1.09 nm
D) 1820 nm
E) 0.0123 m

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
34
_____ is the frequency of light below which no electrons are emitted regardless of the light's intensity.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
35
According to the Niels Bohr's model of atom, the orbits of electrons are determined purely by the electrostatic forces within the atom.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
36
The _____ refers to the sharp decrease in the intensity of black body radiation emitted at shorter wavelengths.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
37
A(n) _____ is a quantum of radiant energy each with a particular energy E.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
38
Max Planck proposed that the energy of electromagnetic waves is continuous rather than quantized.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
39
Briefly explain the photoelectric effect. What is threshold frequency?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
40
To explain the line emission spectra of hydrogen in the visible region, Balmer proposed the following formula 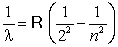 . According to this formula, which of the following is the possible value for n?
. According to this formula, which of the following is the possible value for n?
A) 1
B) 2
C) 3
D) -1
E) -3
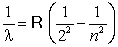 . According to this formula, which of the following is the possible value for n?
. According to this formula, which of the following is the possible value for n?A) 1
B) 2
C) 3
D) -1
E) -3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
41
The minimum uncertainty in the position of a tennis ball weighting 125.0 g and traveling at a speed of 70.00 1.000 mi/h is _____.
A) 6.626
B) 9.436
C) 1.666
D) 0.5345
E) 1.310
A) 6.626
B) 9.436
C) 1.666
D) 0.5345
E) 1.310

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
42
Explain the working of a laser with an example.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
43
The wavelength of a tennis ball weighing 70.0 g and travelling at a speed of 40.0 miles/hour is _____.
A) 6.63 × 1034 m
B) 1.252 × 1034 m
C) 5.29 × 10-34 m
D) 0.371 × 10-34 m
E) 9.46 × 10-34 m
A) 6.63 × 1034 m
B) 1.252 × 1034 m
C) 5.29 × 10-34 m
D) 0.371 × 10-34 m
E) 9.46 × 10-34 m

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
44
Explain Bohr's model of the hydrogen atom.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
45
What is the energy of the radiation required to excite an atom from the n1=1 orbit to the n2=4 orbit?
A) 1230 kJ/mol of photon
B) 3120 kJ/mol of photon
C) 122 kJ/mol of photon
D) 656 kJ/mol of photon
E) 434 kJ/mol of photon
A) 1230 kJ/mol of photon
B) 3120 kJ/mol of photon
C) 122 kJ/mol of photon
D) 656 kJ/mol of photon
E) 434 kJ/mol of photon

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
46
A(n)_____ is the point at which the amplitude of the wave is zero.
A) overtone
B) node
C) standing wave
D) fundamental point
E) still zone
A) overtone
B) node
C) standing wave
D) fundamental point
E) still zone

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
47
The energy level in an atom corresponding to n=5 will have a total of 5 nodes.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
48
A(n) _____ spectrum is a spectrum produced by the emission of light by atoms in excited states.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
49
A node is the point where the amplitude of a wave is zero.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
50
What is the de Broglie wavelength of a 20,000.0 pound jet fighter traveling at 1200.0 km/hr?
A) 0.1430
B) 2.191
C) 2.191
D) 1.879
E) 1.879
A) 0.1430
B) 2.191
C) 2.191
D) 1.879
E) 1.879

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
51
In the emission spectrum of hydrogen, the Balmer series of lines corresponds to transition of electrons from the excited states to the n1=1 orbit. What is the wavelength of the energy line n2=4 in the Balmer series?
A) 97.2 nm
B) 486 nm
C) 434 nm
D) 1870 nm
E) 65.6 nm
A) 97.2 nm
B) 486 nm
C) 434 nm
D) 1870 nm
E) 65.6 nm

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
52
The Paschen series are due to transitions from higher-energy orbits to orbits with _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
53
What is the wavelength of a neutron (1.675 × 10-27 kg) that is moving at 1.400 × 10-7 m/s?
A) 3.96 × 107 m
B) 7.98 × 10-7 m
C) 2.83 m
D) 4.00 m
E) 4.50 × 10-7 m
A) 3.96 × 107 m
B) 7.98 × 10-7 m
C) 2.83 m
D) 4.00 m
E) 4.50 × 10-7 m

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
54
A standing circular wave has a circumference of 2.734 units. Which of the following wavelengths would cause destructive interference of the wave?
A) 2.734 units
B) 5.468 units
C) 8.202 units
D) 12.303 units
E) 21.872 units
A) 2.734 units
B) 5.468 units
C) 8.202 units
D) 12.303 units
E) 21.872 units

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
55
The _____ state is the most stable arrangement of electrons for an element or a compound.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
56
A(n) _____ is any arrangement of electrons that is higher in energy than the ground state.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
57
The minimum uncertainty in the position of a neutron travelling at a speed of 2.2 km/s ± 0.1% is _____. Given: The weight of a neutron is 1.675 * 10-27 kg.
A) 0.1430
B) 0.1430
C) 0.3106
D) 0.3106
E) 0.1430
A) 0.1430
B) 0.1430
C) 0.3106
D) 0.3106
E) 0.1430

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
58
What is the energy of the radiation used to excite the hydrogen atom from an n1=2 state to an n2=5 state?
A) 656 * 10-19 J/photon
B) 9.73 * 10-19 J/photon
C) 3.97 * 10-19 J/photon
D) 8.54 * 10-19 J/photon
E) 4.58 * 10-19 J/photon
A) 656 * 10-19 J/photon
B) 9.73 * 10-19 J/photon
C) 3.97 * 10-19 J/photon
D) 8.54 * 10-19 J/photon
E) 4.58 * 10-19 J/photon

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
59
A(n) _____ is a spectrum produced by the absorption of light by ground-state atoms.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
60
An overtone is the lowest-energy standing wave.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
61
If the maximum value for the Azimuthal quantum number in an atom is 5, then the principal quantum number is _____.
A) 4
B) -5
C) 0
D) -1
E) 6
A) 4
B) -5
C) 0
D) -1
E) 6

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
62
What is the de Broglie wavelength of an electron traveling at a velocity of 4.53 × 106 m/s in a hydrogen atom? Given: Mass of an electron: 9.11 × 10-31 kilograms
A) 2.26 × 10-34 m
B) 9.10 × 10-31 m
C) 4.78 × 10-11 m
D) 6.74 × 10-14 m
E) 1.61 × 10-10 m
A) 2.26 × 10-34 m
B) 9.10 × 10-31 m
C) 4.78 × 10-11 m
D) 6.74 × 10-14 m
E) 1.61 × 10-10 m

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
63
Quantum mechanics predicts that in the hydrogen atom, all orbitals with the same value of n (e.g., the three 2p orbitals) are degenerate, meaning:
A) that they have similar looking shapes.
B) that they are similarly oriented in space.
C) that they have the same frequency.
D) that they have the same wavelength.
E) that they have the same energy.
A) that they have similar looking shapes.
B) that they are similarly oriented in space.
C) that they have the same frequency.
D) that they have the same wavelength.
E) that they have the same energy.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
64
A wave function (Ψ) relates the location of an electron at a given point in space to the amplitude of its wave.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following quantum numbers describes the orientation of the electron distribution in an atom?
A) n
B) s
C) l
D) k
E) ml
A) n
B) s
C) l
D) k
E) ml

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
66
In Bohr's model of the hydrogen atom, an electron behaves like a(n) _____, a wave which does not travel through space.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
67
An electron in the outermost shell of a(n) _____ atom would experience the most amount of shielding.
A) chlorine
B) fluorine
C) iodine
D) hydrogen
E) astatine
A) chlorine
B) fluorine
C) iodine
D) hydrogen
E) astatine

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
68
If the position of a particle is known absolutely, then the uncertainty in the momentum of the particle must be _____.
A) equal to its mass
B) equal to the Planck's constant
C) infinite
D) equal to the velocity of the particle
E) zero
A) equal to its mass
B) equal to the Planck's constant
C) infinite
D) equal to the velocity of the particle
E) zero

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
69
Write a note on de Broglie's explanation of constructive and destructive interference.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following 3d orbitals has a distinct shape?
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
71
The azimuthal quantum number (l) gives the average relative distance of an electron from the nucleus.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
72
If the azimuthal quantum number is 0, the magnetic quantum number can be only 0.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
73
s orbitals become larger as they extend farther from the nucleus.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
74
How many orbitals are in the principal shell with n = 2?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
75
A(n) _____ is the point where the amplitude of a wave is zero.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
76
According to de Broglie, an electron can be described by a wave whose wavelength is given by _____.
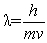
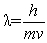

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
77
A(n) _____ is the vibration of a standing wave that is higher in energy than the fundamental vibration.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
78
The _____ is a principle that matter and energy have properties typical of both waves and particles.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
79
A 3px orbital will have the same shape, size, and energy level as the _____ orbital.
A) 2s
B) 3s
C) 3py
D) 4px
E) 4py
A) 2s
B) 3s
C) 3py
D) 4px
E) 4py

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
80
Write a note on Heisenberg's uncertainty principle.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck


