Deck 2: Molecules, Ions, and Chemical Formulas
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/110
Play
Full screen (f)
Deck 2: Molecules, Ions, and Chemical Formulas
1
Which of the following compounds has only covalent bonding?
A) H2O
B) NaOH
C) NaCl
D) Al2(SO3)3
E) Fe2O3
A) H2O
B) NaOH
C) NaCl
D) Al2(SO3)3
E) Fe2O3
H2O
2
Cl- is a(n) _____ ion.
monoatomic
3
Differentiate between organic and inorganic compounds.
Covalent compounds that contain predominantly carbon and hydrogen are called organic compounds. Compounds that consist primarily of elements other than carbon and hydrogen are called inorganic compounds; they include both covalent and ionic compounds.
4
Which of the following is an elemental-polyatomic molecule?
A) Carbon tetrachloride
B) Nitrogen
C) Phosphorous
D) Sodium chloride
E) Chlorine
A) Carbon tetrachloride
B) Nitrogen
C) Phosphorous
D) Sodium chloride
E) Chlorine

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
5
How many electrons are shared between two atoms joined by a double bond?
A) One
B) Two
C) Four
D) Six
E) Eight
A) One
B) Two
C) Four
D) Six
E) Eight

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
6
In a(n) _____, the atoms are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons they share.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
7
In an ionic bond, the atoms are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons they share.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
8
What is a chemical bond? Briefly describe the types of chemical bonds.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
9
If two charged particles, Q1 and Q2, are separated by the distance r, the electrostatic energy between the particles is _____
A) Q1× Q2 + r
B) Q1× Q2× r
C)
D)
E)
A) Q1× Q2 + r
B) Q1× Q2× r
C)
D)
E)

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
10
Methane (CH4) is a covalent molecule, where electrons are shared between the carbon and hydrogen atoms.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
11
Each covalent compound is represented by a(n) _____ , which gives the atomic symbol for each component element, in a prescribed order, accompanied by a subscript indicating the number of atoms of that element in the molecule.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an organic compound?
A) FeO
B) NaHCO3
C) CH4
D) SF6
E) H2CO3
A) FeO
B) NaHCO3
C) CH4
D) SF6
E) H2CO3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
13
Which method of representing the structure of a molecule uses wedges and dashes to sketch the structure of a molecule in three dimensions?
A) Ball-and-stick model
B) Perspective drawing
C) Space filling model
D) Condensed structural formula
E) Molecular formula
A) Ball-and-stick model
B) Perspective drawing
C) Space filling model
D) Condensed structural formula
E) Molecular formula

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following molecules has a tetrahedral geometry?
A) H2O
B) CO2
C) CH4
D) NO2
E) NH3
A) H2O
B) CO2
C) CH4
D) NO2
E) NH3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
15
Ions that contain more electrons than protons have a net negative charge and are called _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
16
The most common monoatomic ion formed by magnesium is _____.
A) Mg-
B) Mg2-
C) Mg3+
D) Mg2+
E) Mg3-
A) Mg-
B) Mg2-
C) Mg3+
D) Mg2+
E) Mg3-

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
17
There is a triple bond between the two oxygen atoms in a molecule of O2.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
18
A Ca2+ ion has the same number of electrons as the noble gas Argon.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
19
What is the total number of electrons present in an Mg2+ ion?
A) 8
B) 10
C) 12
D) 14
E) 16
A) 8
B) 10
C) 12
D) 14
E) 16

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
20
Ions that contain fewer electrons than protons have a net positive charge and are called anions.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
21
The thiocyanate ion (SCN-) is an example of a polyatomic anion.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is the compound formed from the ions NH4+ and NO3-?
A) N2O2H2
B) HNO3
C) NO4NH3
D) NH3NO4
E) NH4NO3
A) N2O2H2
B) HNO3
C) NO4NH3
D) NH3NO4
E) NH4NO3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
23
_____ are compounds that contain specific ratios of loosely bound water molecules.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
24
HSO4- is an example of a polyatomic _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
25
A dichromate ion, Cr2O72−, will react with _____ potassium ions to form potassium dichromate.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is the formula of the polyatomic ion peroxide?
A)
B)
C)
D) .
E)
A)
B)
C)
D) .
E)

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
27
The compound formed from Fe3+ and O2- is_____.
A) FeO
B) Fe2O
C) FeO2
D) FeO3
E) Fe2O3
A) FeO
B) Fe2O
C) FeO2
D) FeO3
E) Fe2O3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
28
What are polyatomic ions? Give an example of a polyatomic anion and a polyatomic cation.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
29
The _____ of a compound is the relative number of atoms of the elements in a compound, reduced to the smallest whole numbers.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
30
An ionic compound that contains only two elements, one present as a cation and one as an anion, is called a(n) _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
31
Sodium oxide (Na2O) is a binary ionic compound.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is the compound formed from the ions Pb2+ and S2-?
A) PbS
B) Pb2S
C) PbS2
D) 2Pb2S
E) Pb2S2
A) PbS
B) Pb2S
C) PbS2
D) 2Pb2S
E) Pb2S2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
33
The ion pair Na+ and SO42- forms the compound Na4SO.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is the compound formed from the ions Cu+ and O2-?
A) CuO
B) Cu2O
C) CuO2
D) CuO3
E) Cu2O3
A) CuO
B) Cu2O
C) CuO2
D) CuO3
E) Cu2O3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
35
The systematic name for cuprous chloride is copper (II) chloride.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
36
Two chlorine ions react with one chromium (II) ion to form chromic chloride.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
37
The empirical formula for the compound formed from an aluminum ion and a sulfate ion is _____.
A) AlSO4
B) Al(SO4)2
C) Al2SO4
D) (AlSO4)2
E) Al2(SO4)3
A) AlSO4
B) Al(SO4)2
C) Al2SO4
D) (AlSO4)2
E) Al2(SO4)3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
38
Chlorine with atomic number 17 would most likely _____ an electron, during ionic bonding, to attain the noble gas configuration of Argon.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
39
The formula unit of a compound is the relative numbers of atoms of the elements in a compound, reduced to the smallest whole numbers.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
40
The dichromate ion (Cr2O7-) is an example of a polyatomic cation.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
41
The ion is called _____.
A) per chromate
B) chromite
C) chromium oxate
D) chromate
E) dichromate
A) per chromate
B) chromite
C) chromium oxate
D) chromate
E) dichromate

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
42
What is the systematic name for FeS?
A) Ferric sulfide
B) Iron(I) sulfide
C) Iron (II) sulfide
D) Ferrous sulfide
E) Iron (III) sulfide
A) Ferric sulfide
B) Iron(I) sulfide
C) Iron (II) sulfide
D) Ferrous sulfide
E) Iron (III) sulfide

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
43
The systematic name of Mg(HCO3)2 is _____.
A) magnesium carbonate
B) magnesium bicarbonate
C) magnesium formate
D) magnesium acetate
E) manganese (II) bicarbonate
A) magnesium carbonate
B) magnesium bicarbonate
C) magnesium formate
D) magnesium acetate
E) manganese (II) bicarbonate

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
44
The systematic name for OF2 is oxygen difluoride.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
45
Cyclohexane is a saturated hydrocarbon.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
46
Explain the different conventions involved in naming a polyatomic anion?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
47
(NH4)2SO4 is called _____ in the systematic nomenclature.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
48
The common or old name for iron (II) sulfide is _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
49
Polyatomic anions that contain a single metal or nonmetal atom plus one or more oxygen atoms are called _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
50
Cyclopropane has four carbon atoms in its structure.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
51
The formula for the sulfite ion is _____.
A)
B)
C)
D)
E)
A)
B)
C)
D)
E)

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
52
NaClO4 is referred to as sodium hypochlorite.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
53
The common name for the compound Fe2(SO4)3 is _____.
A) iron sulfate
B) ferric sulfate
C) iron-two sulfate
D) ferrous sulfate
E) iron-three sulfate
A) iron sulfate
B) ferric sulfate
C) iron-two sulfate
D) ferrous sulfate
E) iron-three sulfate

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
54
NaC2H3O2 is called sodium acetate.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
55
The formula for calcium formate is ____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
56
The formula for sodium bicarbonate is _____.
A) NaCO2
B) Na2CO3
C) Na2HCO3
D) NaHCO3
E) Na2(CO3)2
A) NaCO2
B) Na2CO3
C) Na2HCO3
D) NaHCO3
E) Na2(CO3)2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
57
C6H5CH2OH is the condensed molecular formula of phenol.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
58
The formula of nitrogen dioxide is N2O2.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
59
What is the systematic name of NH4ClO4?
A) Chlorine nitride
B) Ammonium chlorate
C) Ammonium chloride
D) Chlorammonium hydride
E) Ammonium perchlorate
A) Chlorine nitride
B) Ammonium chlorate
C) Ammonium chloride
D) Chlorammonium hydride
E) Ammonium perchlorate

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
60
The formula for aluminum sulfite is _____.
A) AlSO3
B) Al2(SO3)2
C) Al2(SO4)3
D) Al3(SO3)2
E) Al2(SO3)3
A) AlSO3
B) Al2(SO3)2
C) Al2(SO4)3
D) Al3(SO3)2
E) Al2(SO3)3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
61
The molecular formula for cyclobutane is _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is the formula of niobium (IV) oxide?
A) Nb4O
B) Ni4O
C) NO2
D) N2O2
E) NbO2
A) Nb4O
B) Ni4O
C) NO2
D) N2O2
E) NbO2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is the condensed structural formula for isobutane?
A) CH3CH2CH2CH3.
B) CH3CH3CH2CH2
C) CH3CH2CH2CH3
D) (CH3)3CH2
E) (CH3)2CHCH3
A) CH3CH2CH2CH3.
B) CH3CH3CH2CH2
C) CH3CH2CH2CH3
D) (CH3)3CH2
E) (CH3)2CHCH3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
64
Calcium oxide when dissolved in water results in a basic solution.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
65
Sulfuric acid is an example of an oxoacid.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
66
What is the name of RbS?
A) Sulfo rhenium
B) Rhodium sulfide
C) Sulfo ruthenium
D) Ruthenium sulfide
E) Rubidium sulfide
A) Sulfo rhenium
B) Rhodium sulfide
C) Sulfo ruthenium
D) Ruthenium sulfide
E) Rubidium sulfide

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
67
HClO is the formula of perchloric acid.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
68
The formula of copper (I) oxide is _____.
A) CuO
B) Cu2O
C) Co2O2
D) Cu3O2
E) CoO
A) CuO
B) Cu2O
C) Co2O2
D) Cu3O2
E) CoO

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
69
CH3CH2CH2CH2CH3 is called _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
70
What is the name of CH3C≡CCH3?
A) 2-butane
B) 2-butene
C) 2-pentene
D) 2-butyne
E) 2-cyclobutane
A) 2-butane
B) 2-butene
C) 2-pentene
D) 2-butyne
E) 2-cyclobutane

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
71
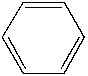
Which of the following is the structure of toluene?
A)
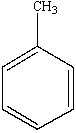
B)
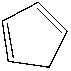
C)
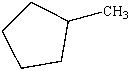
D)
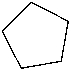
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
72
Briefly describe the four major classes of hydrocarbons. What are saturated and unsaturated hydrocarbons?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
73
Unsaturated hydrocarbons are those which contain multiple bonds such as alkenes and _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
74
What is the name of Tl2Se?
A) Titanium (II) sulfate
B) Titanium (I) sulfite
C) Terillium (II) selenide
D) Thallium (I) selenide
E) Tungsten (II) sulfide
A) Titanium (II) sulfate
B) Titanium (I) sulfite
C) Terillium (II) selenide
D) Thallium (I) selenide
E) Tungsten (II) sulfide

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
75
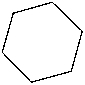
Which of the following is the structure of cyclobutane?
A)
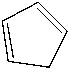
B)
C)
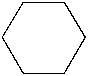
D)

E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
76
_____ are the simplest class of organic compounds, consisting entirely of carbon and hydrogen.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
77
Chlorous acid is the acid formed by adding one proton to the chlorite ion (ClO2−).

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
78
What are cyclic hydrocarbons? Give examples of cyclic hydrocarbons. Explain how to draw cyclic hydrocarbons.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is the simplest alkene?
A) C2H6
B) C6H6
C) C2H4
D) C2H2
E) C6H12
A) C2H6
B) C6H6
C) C2H4
D) C2H2
E) C6H12

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
80
The molecular formula of ethanol is _____.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck


