Deck 5: Energy Changes in Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/93
Play
Full screen (f)
Deck 5: Energy Changes in Chemical Reactions
1
The work done by a person while moving a 15.0 kg sphere at an acceleration of 2.0 m/s2 to a store at a distance of 6.0 meters from its original position is _____J.
A) 1.8 × 102
B) 0.9 × 102
C) 1.8 × 101
D) 18 × 102
E) 2.5 × 102
A) 1.8 × 102
B) 0.9 × 102
C) 1.8 × 101
D) 18 × 102
E) 2.5 × 102
1.8 × 102
2
The energy contained in batteries is an example of chemical energy.
True
3
The negative sign associated with the pressure-volume work done indicates that the system gains energy.
False
4
The force exerted by gravity on a 25 lb object lifted to a height of 6.0ft above the ground is _____ N. ( Given work done = 2.0 × 102 J)
A) 1.1 × 102
B) 1.8 × 102
C) 11 × 102
D) 2.0 × 102
E) 2.4 × 102
A) 1.1 × 102
B) 1.8 × 102
C) 11 × 102
D) 2.0 × 102
E) 2.4 × 102

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
5
What is the SI unit of energy?
A) Fahrenheit
B) Joules
C) Newton
D) Pascal
E) Ohm
A) Fahrenheit
B) Joules
C) Newton
D) Pascal
E) Ohm

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
6
The energy that results from atomic or molecular motion is called _____.
A) radiant energy
B) chemical energy
C) nuclear energy
D) thermal energy
E) electrical energy
A) radiant energy
B) chemical energy
C) nuclear energy
D) thermal energy
E) electrical energy

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
7
Nuclear energy is a form of a potential energy.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
8
The _____ of an object is a measure of its thermal energy content.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
9
The energy that is stored in an object because of its relative positions or orientations of its components is called _____.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
10
A closed system can exchange energy but not matter with its surroundings.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
11
What are the factors that influence the amount of work done while moving an object?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
12
Electrical energy is the energy carried by light, microwaves, and radio waves.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
13
The kinetic energy of a bicycle that weighs 23.5 kg moving at a speed of 25.0 miles per hour is _____ J.
A) 5.87 × 103
B) 2.93 × 103
C) 2.68 × 103
D) 0.29 × 103
E) 1.47 × 103
A) 5.87 × 103
B) 2.93 × 103
C) 2.68 × 103
D) 0.29 × 103
E) 1.47 × 103

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following can be categorized as potential energy?
A) The energy released during a landslide
B) The energy associated with a bullet in a gun
C) The energy associated with a moving bicycle
D) The energy associated with a swinging pendulum
E) The energy released when kicking a ball
A) The energy released during a landslide
B) The energy associated with a bullet in a gun
C) The energy associated with a moving bicycle
D) The energy associated with a swinging pendulum
E) The energy released when kicking a ball

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
15
If the enthalpy of a reaction is negative, then the enthalpy of the products is greater than the enthalpy of the reactants.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is an example of radiant energy?
A) Energy contained in lightning
B) Energy stored in petrol
C) Energy obtained from sunlight
D) Energy contained in boiling water
E) Energy emitted from radioactive materials
A) Energy contained in lightning
B) Energy stored in petrol
C) Energy obtained from sunlight
D) Energy contained in boiling water
E) Energy emitted from radioactive materials

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
17
_____ is the thermal energy that can be transferred from an object at one temperature to an object at another temperature.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
18
Explain with an example, how potential energy is transformed into kinetic energy.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
19
The potential energy associated with a person weighing 78 kg standing at the edge of a diving board 4.0 ft above the water level is _____ 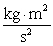 .
.
A) 3.0 × 102
B) 5.9 × 103
C) 9.3 × 103
D) 9.3 × 102
E) 5.9 × 102
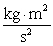 .
.A) 3.0 × 102
B) 5.9 × 103
C) 9.3 × 103
D) 9.3 × 102
E) 5.9 × 102

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
20
Different forms of energy are expressed in different units.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
21
If ΔT and q values are positive, then the heat flows from the surroundings into an object.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
22
The magnitude of thermal change of an object depends only on the amount of thermal energy transferred.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
23
Bomb calorimeter is a constant-pressure calorimeter.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
24
The standard enthalpy of formation of any element in its most stable state is zero.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
25
_____ is an example of a state function.
A) Pressure-volume work
B) Mechanical work
C) Heat
D) Pressure
E) Distance
A) Pressure-volume work
B) Mechanical work
C) Heat
D) Pressure
E) Distance

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
26
A(n) _____ is a property of a system whose magnitude depends only on the present state of the system, not its previous history.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
27
The amount of heat absorbed or released by the calorimeter is equal in magnitude and opposite in sign to the amount of heat produced or consumed by the reaction.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
28
The enthalpy change for a reaction depends on the path by which the reactants are converted to products.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
29
A(n) _____ system exchanges neither energy nor matter with the surroundings.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
30
The balanced chemical equation corresponding to the standard enthalpy of formation of NaCl is:
A) Na2(s) + Cl2(g) → 2NaCl(s).
B) 2Na(s) + Cl2(g) → 2NaCl(s).
C) Na(s) + Cl2 (g)→ NaCl(s).
D) Na(s) + ½ Cl2(g)→ NaCl(s).
E) Na(s) + Cl(g)→ NaCl(s).
A) Na2(s) + Cl2(g) → 2NaCl(s).
B) 2Na(s) + Cl2(g) → 2NaCl(s).
C) Na(s) + Cl2 (g)→ NaCl(s).
D) Na(s) + ½ Cl2(g)→ NaCl(s).
E) Na(s) + Cl(g)→ NaCl(s).

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
31
In the equation ΔE = q + w, the symbol E represents the _____.
A) heat
B) temperature
C) enthalpy
D) internal energy
E) work done
A) heat
B) temperature
C) enthalpy
D) internal energy
E) work done

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
32
The small well-defined part of a universe in which we are interested is referred to as a(n) _____.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
33
In an exothermic process:
A) the volume of the system decreases at constant pressure.
B) the heat is transferred to the system from its surroundings.
C) the value of q is less than zero by convention.
D) the enthalpy of products is greater than the enthalpy of the reactants.
E) the enthalpy of the reaction is positive.
A) the volume of the system decreases at constant pressure.
B) the heat is transferred to the system from its surroundings.
C) the value of q is less than zero by convention.
D) the enthalpy of products is greater than the enthalpy of the reactants.
E) the enthalpy of the reaction is positive.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
34
Define standard enthalpies of formation.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
35
The _____ of a system is a complete description of the system at a given time, including its temperature and pressure, the amount of matter it contains, its chemical composition, and the physical state of the matter.
A) internal energy
B) state
C) enthalpy
D) work
E) entropy
A) internal energy
B) state
C) enthalpy
D) work
E) entropy

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
36
The enthalpy change that occurs when a reaction is carried out with all reactants and products in their standard state is known as the _____.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
37
The units of specific heat (Cs) are J/(mol.0°C)

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
38
A process in which heat is transferred from a system to its surroundings is described as _____.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O (l). The ΔHocomb of methane is _____ kJ/mol.
(Given ΔHof values of methane, carbon dioxide, and water is -74.6, -393.5, and -285.8 kJ/mol respectively)
A) -890.5
B) -799.8
C) -1033
D) 890.5
E) 799.8
(Given ΔHof values of methane, carbon dioxide, and water is -74.6, -393.5, and -285.8 kJ/mol respectively)
A) -890.5
B) -799.8
C) -1033
D) 890.5
E) 799.8

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
40
State Hess's law. How can it be used to calculate the enthalpy change of a reaction that cannot be observed directly?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
41
The amount of heat absorbed by 500.0 kg sodium chloride used in a thermal energy storage unit, when the temperature of the salt increases from 15.0 oC to 38.0oC is _____. (Given, Cs of NaCl = 0.864 J/g.oC)
A) 9.94 × 103 kJ
B) 9.94 × 106 kJ
C) 1.64 × 103 kJ
D) 1.64 × 104 kJ
E) 8.63 × 106 kJ
A) 9.94 × 103 kJ
B) 9.94 × 106 kJ
C) 1.64 × 103 kJ
D) 1.64 × 104 kJ
E) 8.63 × 106 kJ

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following has the highest heat capacity?
A) Methanol
B) Benzene
C) Graphite
D) Liquid water
E) Water vapour
A) Methanol
B) Benzene
C) Graphite
D) Liquid water
E) Water vapour

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
43
The reported caloric content of foods does not include the ΔHcomb of _____.
A) carbohydrates
B) vitamins
C) proteins
D) fats
E) minerals
A) carbohydrates
B) vitamins
C) proteins
D) fats
E) minerals

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
44
Define heat capacity. What are the factors that determine the heat capacity of an object?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
45
Human body usually exhausts the supply of stored carbohydrates before utilizing the fat reserves.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
46
Fiber contributes to the caloric content of food in humans.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is an essential amino acid?
A) Glycine
B) Threonine
C) Alanine
D) Serine
E) Aspartic acid
A) Glycine
B) Threonine
C) Alanine
D) Serine
E) Aspartic acid

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following devices is best suited to measure the enthalpy changes that accompany combustion reactions?
A) Coffee-cup calorimeter
B) Constant-pressure calorimeter
C) Bomb calorimeter
D) Heat balance calorimeter
E) Constant flux calorimeter
A) Coffee-cup calorimeter
B) Constant-pressure calorimeter
C) Bomb calorimeter
D) Heat balance calorimeter
E) Constant flux calorimeter

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
49
The number of calories expended by a 60.0 kg person bicycling at 15.0 miles per hour is _____ kcal.
A) 3.22 × 10-1
B) 2.32 × 10-1
C) 6.44 × 10-1
D) 4.46 × 10-1
E) 5.46 × 10-1
A) 3.22 × 10-1
B) 2.32 × 10-1
C) 6.44 × 10-1
D) 4.46 × 10-1
E) 5.46 × 10-1

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
50
How are the enthalpy changes that accompany combustion reactions measured using a bomb calorimeter?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
51
_____ is the amount of energy needed to raise the temperature of an object exactly by 1°C.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
52
The caloric content of a meal that contains 81.5 g of water, 76.8 g of protein, 25.8 g of fat, 12.0 g of carbohydrates, and 2.0 g of minerals is approximately _____ Cal.
A) 842
B) 587
C) 971
D) 1030
E) 902
A) 842
B) 587
C) 971
D) 1030
E) 902

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
53
A device used to measure enthalpy changes in chemical processes at constant pressure is _____.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
54
A glass of milk contains 85.0 g of water, 5.0 g of carbohydrates, 3.0 g of fats, and 5.0 g of proteins. The caloric content of milk is _____ Cal.
A) 92
B) 77
C) 67
D) 52
E) 65
A) 92
B) 77
C) 67
D) 52
E) 65

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
55
The nitrogen in foods, when burned in a calorimeter is transformed into urea.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
56
In the equation q = mCsΔT, Cs represents _____.
A) heat capacity
B) specific heat
C) change in enthalpy
D) state function
E) molar heat capacity
A) heat capacity
B) specific heat
C) change in enthalpy
D) state function
E) molar heat capacity

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
57
The structure given below is the structure of _____. 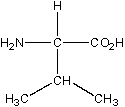
A) alanine
B) glycine
C) valine
D) lysine
E) serine

A) alanine
B) glycine
C) valine
D) lysine
E) serine

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
58
The enthalpy of combustion is negative for all the substances that can be burned.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
59
_____ describes a set of techniques employed to measure enthalpy changes in chemical processes using calorimeters.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
60
The _____ is the amount of energy needed to increase the temperature of 1 mol of a substance by 1°C.
A) specific heat
B) molar heat capacity
C) enthalpy
D) state function
E) change in enthalpy
A) specific heat
B) molar heat capacity
C) enthalpy
D) state function
E) change in enthalpy

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
61
The amount of energy released during the combustion of 0.018 gal of ethanol that has a density of 0.78 g/mL is _____ kJ. (Given ΔHocomb of ethanol = -1371 kJ/mol).
A) -2.0 × 103
B) -3.0 × 103
C) -4.5 × 103
D) -0.5 × 103
E) -1.6 × 103
A) -2.0 × 103
B) -3.0 × 103
C) -4.5 × 103
D) -0.5 × 103
E) -1.6 × 103

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
62
What is syngas?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
63
_____ is a unit used to indicate the caloric content of food.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
64
Anthracite has high sulfur content because of the presence of small particles of pyrite.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
65
How are the caloric values reported for foods different from the enthalpy of combustion of the same foods burned in a calorimeter?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
66
Coal contains large number of _____ atoms in their structure that link parts of the structure together, in addition to the basic framework of carbon-carbon bonds.
A) hydrogen
B) sulfur
C) oxygen
D) helium
E) nitrogen
A) hydrogen
B) sulfur
C) oxygen
D) helium
E) nitrogen

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
67
What are the different approaches that can be used to determine the caloric content of foods?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
68
_____ is the partially decayed remains of plants that grow in swampy areas and gives off little heat when burnt.
A) Anthracite
B) Graphite
C) Peat
D) Lignite
E) Bituminous coal
A) Anthracite
B) Graphite
C) Peat
D) Lignite
E) Bituminous coal

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
69
The amino acids that a human body is unable to synthesize from the other compounds are called _____.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
70
_____ is a type of coal that has the lowest oxygen content and the highest ΔHcomb.
A) Bituminous
B) Subbituminous
C) Steam coal
D) Lignite
E) Anthracite
A) Bituminous
B) Subbituminous
C) Steam coal
D) Lignite
E) Anthracite

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following types of coal has high oxygen content and the lowest ΔHcomb?
A) Anthracite
B) Lignite
C) Subbituminous
D) Bituminous
E) Graphite
A) Anthracite
B) Lignite
C) Subbituminous
D) Bituminous
E) Graphite

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
72
The hydrogen:carbon mole ratio in lignite is _____.
A) 0.5
B) 0.6
C) 0.8
D) 1.0
E) 0.9
A) 0.5
B) 0.6
C) 0.8
D) 1.0
E) 0.9

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
73
How is coal converted to liquid fuels?

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the equation C2H5OH + 3O2 → 2CO2 + 3H2O. The ΔHocomb for the given reaction is _____.
(Given ΔHof values of C2H5OH = -277.60 kJ/mol, CO2 = -393.50 kJ/mol, and H2O = -285.30 kJ/mol)
A) -1920.5 kJ
B) 1365.3 kJ
C) -1365.3 kJ
D) -1642.9 kJ
E) 1920.5 kJ
(Given ΔHof values of C2H5OH = -277.60 kJ/mol, CO2 = -393.50 kJ/mol, and H2O = -285.30 kJ/mol)
A) -1920.5 kJ
B) 1365.3 kJ
C) -1365.3 kJ
D) -1642.9 kJ
E) 1920.5 kJ

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
75
In the human body, the nitrogen from foods is converted to _____ before excretion.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
76
Primary sources of energy like natural gas are more efficient when used directly than in the form of a secondary source such as electricity.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
77
The structure of coal is more similar to an aliphatic hydrocarbon structure than an aromatic hydrocarbon.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
78
The energy required by a 55 kg person to climb a 1-story building (Assume each flight of stairs is 18 ft high.) is _____ kcal.
A) 2.56
B) 0.0256
C) 0.700
D) 3.51
E) 9.71
A) 2.56
B) 0.0256
C) 0.700
D) 3.51
E) 9.71

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
79
Coal liquefaction techniques are economically less attractive than the production of liquid fuels from petroleum.

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck
80
The amount of energy released by the combustion of 2 moles of benzene is _____kJ. (ΔHocomb of benzene = -41.8 kJ/g)
A) -3.34 × 103
B) -8.36 × 103
C) -6.52 × 103
D) -5.60 × 103
E) -7.21 × 103
A) -3.34 × 103
B) -8.36 × 103
C) -6.52 × 103
D) -5.60 × 103
E) -7.21 × 103

Unlock Deck
Unlock for access to all 93 flashcards in this deck.
Unlock Deck
k this deck


