Deck 4: Reactions in Aqueous Solution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 4: Reactions in Aqueous Solution
1
The relationship between volume and concentration of stock and dilute solutions is .
False
2
When electricity, in the form of an electrical potential, is applied to a solution, ions in solution migrate toward a similarly charged rod or plate to complete an electrical circuit.
False
3
Given: 2C6H5COOH + Na2CO3 2C6H5COONa + H2O + CO2
In the above reaction, the volume of 0.3250 M Na2CO3 needed for the complete reaction of 54.0 g C6H5COOH is17.6 L.
In the above reaction, the volume of 0.3250 M Na2CO3 needed for the complete reaction of 54.0 g C6H5COOH is17.6 L.
False
4
What volume of a 2.00 M sucrose stock solution is necessary to prepare 2,750 mL of a sucrose solution with molarity 5.34 M?
A) 3.01 L
B) 2.10 L
C) 2.00 L
D) 7.34 L
E) 0.750 L
A) 3.01 L
B) 2.10 L
C) 2.00 L
D) 7.34 L
E) 0.750 L

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
Write a short note on parts per million and parts per billion that are used as units to represent concentrations of solutions.
The concentrations of very dilute solutions are often expressed in parts per million (ppm), which is grams of solute per 106 g of solution, or in parts per billion (ppb), which is grams of solute per 109 g of solution. For aqueous solutions at 20°C, 1 ppm corresponds to 1 μg per milliliter, and 1 ppb corresponds to 1 ng per milliliter.
The concentrations of very dilute solutions are often expressed in parts per million (ppm), which is grams of solute per 106 g of solution, or in parts per billion (ppb), which is grams of solute per 109 g of solution. For aqueous solutions at 20°C, 1 ppm corresponds to 1 μg per milliliter, and 1 ppb corresponds to 1 ng per milliliter.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a nonelectrolyte?
A) Acetic acid
B) Methyl ketone
C) Formic acid
D) Sodium chloride
E) Ammonia
A) Acetic acid
B) Methyl ketone
C) Formic acid
D) Sodium chloride
E) Ammonia

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following are the two classes of organic compounds that act as nonelectrolytes?
A) Carboxylic acids and esters
B) Alkanes and carboxylic acids
C) Ketones and aldehydes
D) Alkenes and esters
E) Carboxylic acids and ethers
A) Carboxylic acids and esters
B) Alkanes and carboxylic acids
C) Ketones and aldehydes
D) Alkenes and esters
E) Carboxylic acids and ethers

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
A(n) _____ is a commercially prepared solution of known concentration.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
What is the concentration of OH - in 0.0345 M Ba(OH)2?
A) 0.0251 M
B) 0.0543 M
C) 0.0345 M
D) 0.0960 M
E) 0.0690 M
A) 0.0251 M
B) 0.0543 M
C) 0.0345 M
D) 0.0960 M
E) 0.0690 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
Calcium sulfate is a nonelectrolyte.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
What is the concentration of Ca2+ in 0.0152 M Ca3(NO3)2?
A) 0.0152 M
B) 0.0304 M
C) 0.0456 M
D) 0.456 M
E) 0.304 M
A) 0.0152 M
B) 0.0304 M
C) 0.0456 M
D) 0.456 M
E) 0.304 M

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
Differentiate between strong electrolytes and weak electrolytes.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is an example of a compound that is very soluble in water but that is essentially nonconductive?
A) Sucrose
B) Calcium chloride
C) Barium hydroxide
D) Sulfuric acid
E) Sodium bromide
A) Sucrose
B) Calcium chloride
C) Barium hydroxide
D) Sulfuric acid
E) Sodium bromide

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
Why is water described as a polar substance?

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
_____ are compounds that dissolve in water and have essentially no effect on conductivity.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a strong electrolyte?
A) 2-propanol
B) Formaldehyde
C) Ferrous sulfate
D) Ethanol
E) Acetone
A) 2-propanol
B) Formaldehyde
C) Ferrous sulfate
D) Ethanol
E) Acetone

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
In chemistry, the _____ of a solution describes the quantity of a solute that is contained in a particular quantity of solvent or solution.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
Polar liquids are good solvents for ionic compounds.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
What is an electrolyte?
A) It is the term used to describe acids that do not dissolve in water.
B) It is the term used to describe bases that do not dissolve in water.
C) It is any compound that can form carbon dioxide when it reacts with water.
D) It is any compound that can form ions when it dissolves in water.
E) It is any compound that can act as a solvent for water.
A) It is the term used to describe acids that do not dissolve in water.
B) It is the term used to describe bases that do not dissolve in water.
C) It is any compound that can form carbon dioxide when it reacts with water.
D) It is any compound that can form ions when it dissolves in water.
E) It is any compound that can act as a solvent for water.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
Individual cations and anions that are each surrounded by their own shell of water molecules are called _____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
Given: CH3COOH + NaOH CH3COO−Na+ + H2O
If 663 mL of 0.150 M CH3COOH is mixed with 444 mL of 0.326 M NaOH, the limiting agent in the reaction is ____.
If 663 mL of 0.150 M CH3COOH is mixed with 444 mL of 0.326 M NaOH, the limiting agent in the reaction is ____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
The _____ in the balanced chemical equation tell how many moles of reactants are needed and how many moles of products can be produced.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
Given complete ionic equation: 2Ag+(aq) + 2NO3−(aq) + 2K+(aq) + Cr2O72−(aq) Ag2Cr2O7(s) + 2K+(aq) + 2NO3−(aq) Convert the given equation into an overall chemical equation.
A) 2AgNO3 (aq) + K2Cr2O7 (aq) Ag2Cr2O7(s) + 2KNO3(aq)
B) Ag2Cr2O7(s) + K2Cr2O7 (aq) 2AgNO3 (aq) + 2KNO3(aq)
C) 2AgNO3 (aq) + 2KNO3(aq) Ag2Cr2O7(s) + K2Cr2O7(aq)
D) 2Ag+(aq) + 2NO3−(aq) + 2K+(aq) + Cr2O72−(aq) 2K+(aq) + 2NO3−(aq)
E) 2Ag+(aq) + Cr2O72−(aq) Ag2Cr2O7(s)
A) 2AgNO3 (aq) + K2Cr2O7 (aq) Ag2Cr2O7(s) + 2KNO3(aq)
B) Ag2Cr2O7(s) + K2Cr2O7 (aq) 2AgNO3 (aq) + 2KNO3(aq)
C) 2AgNO3 (aq) + 2KNO3(aq) Ag2Cr2O7(s) + K2Cr2O7(aq)
D) 2Ag+(aq) + 2NO3−(aq) + 2K+(aq) + Cr2O72−(aq) 2K+(aq) + 2NO3−(aq)
E) 2Ag+(aq) + Cr2O72−(aq) Ag2Cr2O7(s)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
The full molecular chemical equation shows all the substances present in the form in which they actually exist in the solution.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
Differentiate between an overall chemical equation and a complete ionic equation.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
Given an outline of the steps involved in the calculation of moles from volume for a given equation.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
Given complete ionic equation: 3Ag+(aq) + 3F−(aq) + 3Na+(aq) + PO43−(aq) → Ag3PO4(s) + 3Na+(aq) + 3F−(aq) In the above complete ionic equation, the spectator ions are:
A) 3Ag+(aq) and 3F−(aq).
B) 3Na+(aq) and 3Ag+(aq).
C) 3F−(aq) and 3Na+(aq).
D) 3Ag+(aq) and (PO4)3−(aq).
E) (PO4)3−(aq) and 3F−(aq).
A) 3Ag+(aq) and 3F−(aq).
B) 3Na+(aq) and 3Ag+(aq).
C) 3F−(aq) and 3Na+(aq).
D) 3Ag+(aq) and (PO4)3−(aq).
E) (PO4)3−(aq) and 3F−(aq).

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
When table salt is added to an aqueous solution of silver nitrate, the exchange reaction yields a precipitate of silver chloride.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
Given: 2C6H5COOH + Na2CO3 2C6H5COONa + H2O + CO2 What mass of CO2 is formed when 275 mL of 0.150 M Na2CO3 is mixed with 775 mL of 0.254 M C6H5COOH?
A) 2.54 g
B) 3.63 g
C) 1.82 g
D) 6.36 g
E) 4.52 g
A) 2.54 g
B) 3.63 g
C) 1.82 g
D) 6.36 g
E) 4.52 g

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is the net ionic equation for the reaction between the aqueous solutions of magnesium sulfate and sodium carbonate?
A) MgSO4(aq) + Na2CO3(aq) → MgCO3(s) + Na2SO4(s)
B) MgSO4(aq) + Na2CO3(aq) → MgCO3(s) + Na+(aq) + SO42-(aq)
C) Na+(aq) + SO42-(aq) → Na2SO4(s)
D) Mg2+(aq) + CO32-(aq)→ MgCO3(s)
E) Mg2+(aq) + SO42-(aq) + Na+(aq) + CO32-(aq) → MgCO3(s) + Na+(aq) + SO42- (aq)
A) MgSO4(aq) + Na2CO3(aq) → MgCO3(s) + Na2SO4(s)
B) MgSO4(aq) + Na2CO3(aq) → MgCO3(s) + Na+(aq) + SO42-(aq)
C) Na+(aq) + SO42-(aq) → Na2SO4(s)
D) Mg2+(aq) + CO32-(aq)→ MgCO3(s)
E) Mg2+(aq) + SO42-(aq) + Na+(aq) + CO32-(aq) → MgCO3(s) + Na+(aq) + SO42- (aq)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
The ions that do not participate in the actual reaction are called _____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
Given: 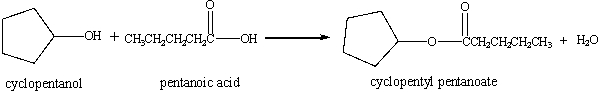 If 885 mL of 0.250 M pentanoic acid(molar mass = 102 g/mol) is mixed with 885 mL of 0.524 M cyclopentanol(molar mass = 86.1 g/mol), the 0.524 M cyclopentanol acts as the limiting agent.
If 885 mL of 0.250 M pentanoic acid(molar mass = 102 g/mol) is mixed with 885 mL of 0.524 M cyclopentanol(molar mass = 86.1 g/mol), the 0.524 M cyclopentanol acts as the limiting agent.
 If 885 mL of 0.250 M pentanoic acid(molar mass = 102 g/mol) is mixed with 885 mL of 0.524 M cyclopentanol(molar mass = 86.1 g/mol), the 0.524 M cyclopentanol acts as the limiting agent.
If 885 mL of 0.250 M pentanoic acid(molar mass = 102 g/mol) is mixed with 885 mL of 0.524 M cyclopentanol(molar mass = 86.1 g/mol), the 0.524 M cyclopentanol acts as the limiting agent.
Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
Calcium hydroxide is insoluble in water.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
_____ is a chemical equation that shows which ions and molecules are hydrated and which are present in other forms and phases.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
Given: HCOOH+ KOH HCOO−K+ + H2O What volume of 0.105 M KOH must be added to 125 mL of a solution containing 6.20 × 10−5 g of HCOOH to ensure that formation of the HCOO−K+ is complete?
A) 18.3 L
B) 6.28 L
C) 125 L
D) 12.8 L
E) 10.5 L
A) 18.3 L
B) 6.28 L
C) 125 L
D) 12.8 L
E) 10.5 L

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
In the equation given below, cyclopentanol (molar mass = 86.1 g/mol) reacts with pentanoic acid (molar mass = 102 g/mol) to produce cyclopentanoate(molar mass = 170.2 g/mol) and water. The volume of 0.297 M pentanoic acid needed for the complete reaction of 40.0 g cyclopentanol is _____ mL. 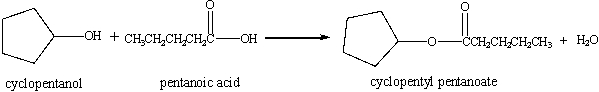
A) 0.86 × 103
B) 5.78 × 103
C) 7.58 × 103
D) 1.56 × 103
E) 0.76 × 103

A) 0.86 × 103
B) 5.78 × 103
C) 7.58 × 103
D) 1.56 × 103
E) 0.76 × 103

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
The net ionic equation shows only those species that participate in the chemical reaction.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
Given: CH3CH2COOH + H2O CH3CH2COO− + H3O+ What volume of 0.500 M CH3CH2COOH is reacting with water to form 2.86 g of CH3CH2COO−?
A) 0.487 L
B) 0.0118 L
C) 0.0487 L
D) 0.0784 L
E) 0.784 L
A) 0.487 L
B) 0.0118 L
C) 0.0487 L
D) 0.0784 L
E) 0.784 L

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
In the reaction of barium chloride with sodium sulfate, SO42- acts as a spectator ion.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
Given: HCOOH + KOH HCOO−K+ + H2O What mass of HCOO−K+ is formed when 383 mL of 0.250 M HCOOH is mixed with 885 mL of 0.524 M KOH?
A) 885 g
B) 48.5 g
C) 8.05 g
D) 1.06 g
E) 32.0 g
A) 885 g
B) 48.5 g
C) 8.05 g
D) 1.06 g
E) 32.0 g

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
The precipitate formed when an aqueous solution of lithium bromide is reacted with an aqueous solution of silver nitrate is _____.
A) LiBr
B) AgBr
C) LiNO3
D) AgNO3
E) LiAg
A) LiBr
B) AgBr
C) LiNO3
D) AgNO3
E) LiAg

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
The insoluble product that forms in a precipitation reaction is called a(n) _____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
HCl is the conjugate acid of the chloride ion.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
Why are precipitation reactions called double displacement reactions?

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
_____ is a monoprotic acid.
A) Sulfuric acid
B) Phosphoric acid
C) Citric acid
D) Benzoic acid
E) Carbonic acid
A) Sulfuric acid
B) Phosphoric acid
C) Citric acid
D) Benzoic acid
E) Carbonic acid

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
What is the pH of a 3.0 × 10−5 M aqueous solution of H2SO4?
A) 4.2
B) 2.4
C) 3.0
D) 0.3
E) -0.3
A) 4.2
B) 2.4
C) 3.0
D) 0.3
E) -0.3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
According to the Arrhenius definition of acids and bases, an acid is a substance:
A) that dissolves in water to produce OH- ions.
B) that accept a proton.
C) that dissolves in water to produce H+ ions.
D) produced by the reaction of two bases.
E) that has a greater OH- ion concentration than H+ ion.
A) that dissolves in water to produce OH- ions.
B) that accept a proton.
C) that dissolves in water to produce H+ ions.
D) produced by the reaction of two bases.
E) that has a greater OH- ion concentration than H+ ion.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is an example of a neutralization reaction?
A) MgCl2+2NaNO3 Mg(NO3)2+2NaCl
B) HI(aq) + KOH(aq) I(s) + H2O(l)
C) 2C6H6COOH + Na2CO3(aq) 2C6H6COO−Na+(aq) + H2O + CO2(g)
D) 2Na+ + S2- → Na2S(s)
E) BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2Na+(aq) + 2Cl-(aq)
A) MgCl2+2NaNO3 Mg(NO3)2+2NaCl
B) HI(aq) + KOH(aq) I(s) + H2O(l)
C) 2C6H6COOH + Na2CO3(aq) 2C6H6COO−Na+(aq) + H2O + CO2(g)
D) 2Na+ + S2- → Na2S(s)
E) BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2Na+(aq) + 2Cl-(aq)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
The net ionic equation for the reaction that occurs when an aqueous solution of lead nitrate is mixed with a solution containing potassium iodide is:
A) K+(aq) + NO3-(aq) → KNO3(s).
B) Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(s).
C) Pb2+(aq) + NO3-(aq) + K+(aq) + 2I-(aq) → KNO3(s) + PbI2(s).
D) Pb2+(aq) + 2I-(aq) → PbI2(s).
E) K+(aq) + I- (aq) → KI(s).
A) K+(aq) + NO3-(aq) → KNO3(s).
B) Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(s).
C) Pb2+(aq) + NO3-(aq) + K+(aq) + 2I-(aq) → KNO3(s) + PbI2(s).
D) Pb2+(aq) + 2I-(aq) → PbI2(s).
E) K+(aq) + I- (aq) → KI(s).

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
_____ is the crystalline solid formed when an aqueous solution of barium chloride is reacted with an aqueous solution of sodium sulfate.
A) NaCl
B) BaCl2
C) Na(OH)2
D) NaSO4
E) BaSO4
A) NaCl
B) BaCl2
C) Na(OH)2
D) NaSO4
E) BaSO4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is an example of a strong base?
A) Acetylacetone
B) Methylamine
C) Ammonia
D) Potassium hydroxide
E) Methylethyl ketone
A) Acetylacetone
B) Methylamine
C) Ammonia
D) Potassium hydroxide
E) Methylethyl ketone

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
Define Arrhenius acids and bases.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
Explain with an example, how precipitation reactions can be used to recover precious metals for recycling.
Student examples may vary.
Precipitation reactions can be used to recover silver from solutions that are used to develop conventional photographic films. Silver bromide turns black when exposed to light and this reaction is used by the photographers to capture images in shades of grey. The washing of unexposed silver bromide using sodium thiosulfate solution generates a dilute silver waste solution. Instant photo operations can generate more than a hundred gallons of dilute silver waste solution per day. The silver from these solutions can be recovered by first removing the thiosulfate by oxidation and then precipitating Ag+ ions with excess chloride ions.
Student examples may vary.
Precipitation reactions can be used to recover silver from solutions that are used to develop conventional photographic films. Silver bromide turns black when exposed to light and this reaction is used by the photographers to capture images in shades of grey. The washing of unexposed silver bromide using sodium thiosulfate solution generates a dilute silver waste solution. Instant photo operations can generate more than a hundred gallons of dilute silver waste solution per day. The silver from these solutions can be recovered by first removing the thiosulfate by oxidation and then precipitating Ag+ ions with excess chloride ions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
CH3CO2H is an example of a polyprotic acid.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following reactions will not result in the formation of a precipitate?
A) Aqueous silver nitrate added to a solution containing potassium chloride
B) Aqueous solution of barium chloride added to a solution containing sodium sulfate
C) Aqueous solution of strontium chloride added to a solution containing potassium carbonate
D) Aqueous solution of sodium sulfate added to a solution containing ammonium iodide
E) Aqueous solution of rubidium hydroxide is added to a solution containing cobalt chloride
A) Aqueous silver nitrate added to a solution containing potassium chloride
B) Aqueous solution of barium chloride added to a solution containing sodium sulfate
C) Aqueous solution of strontium chloride added to a solution containing potassium carbonate
D) Aqueous solution of sodium sulfate added to a solution containing ammonium iodide
E) Aqueous solution of rubidium hydroxide is added to a solution containing cobalt chloride

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
The pH of a solution decreases with the increase in hydrogen ion concentration.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following salts is insoluble in water?
A) (NH4)2SO4
B) (NH4)3PO4
C) LiCl
D) MgSO4
E) Ba(OH)2
A) (NH4)2SO4
B) (NH4)3PO4
C) LiCl
D) MgSO4
E) Ba(OH)2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is a strong acid?
A) HClO
B) HCN
C) HF
D) HNO2
E) HClO4
A) HClO
B) HCN
C) HF
D) HNO2
E) HClO4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
The white precipitate formed when lead acetate is reacted with an aqueous solution of ammonium iodide is _____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is a triprotic acid?
A) Benzoic acid
B) Phosphoric acid
C) Formic acid
D) Nitric acid
E) Acetic acid
A) Benzoic acid
B) Phosphoric acid
C) Formic acid
D) Nitric acid
E) Acetic acid

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
What are the biological effects of acid rain?

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
The _____ ions are responsible for the increased acidity in rain and snow.
A) bromide
B) chloride
C) oxide
D) sulfate
E) phosphate
A) bromide
B) chloride
C) oxide
D) sulfate
E) phosphate

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is an inert metal?
A) Lead
B) Nickel
C) Tin
D) Silver
E) Zinc
A) Lead
B) Nickel
C) Tin
D) Silver
E) Zinc

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
A substance that can behave both as an acid and a base is called a(n) _____ substance.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the reaction: Cu(s) + 2 Ag+(aq) → Cu2+(aq) + 2 Ag(s).
In this reaction, silver is reduced from +1 to 0 oxidation state.
In this reaction, silver is reduced from +1 to 0 oxidation state.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
The compound responsible for the brown color of smog is _____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
Compounds containing elements in low oxidation states become reduced in chemical reactions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
Based on the activity series, cobalt can reduce the salts of _____.
A) chromium
B) cadmium
C) calcium
D) copper
E) iron
A) chromium
B) cadmium
C) calcium
D) copper
E) iron

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
H3O+ is called the _____ ion.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
Metal objects suffer damage from acid rain through oxidation-reduction reactions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
Based on the activity series, there will be no reaction when:
A) lithium metal is dropped into aqueous silver nitrate.
B) a strip of zinc is placed in a solution containing lead iodide.
C) a strip of chromium is placed in a solution containing magnesium sulfate.
D) an iron rod is dipped into a solution of copper sulfate.
E) a chunk of silver is dropped into a solution containing mercuric chloride.
A) lithium metal is dropped into aqueous silver nitrate.
B) a strip of zinc is placed in a solution containing lead iodide.
C) a strip of chromium is placed in a solution containing magnesium sulfate.
D) an iron rod is dipped into a solution of copper sulfate.
E) a chunk of silver is dropped into a solution containing mercuric chloride.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
Differentiate between strong acids and weak acids.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
The balanced chemical equation for the reaction when molten iron reacts with water to form ferrous ions and hydrogen gas is:
A) 2Fe(l) + 2H2O(l) → Fe2+(aq) + 3OH-(aq) + 2H2(g)
B) Fe(l) + 2H2O(l) → Fe2+(aq) + H2(g)
C) 2Fe(l) + H2O(l) → Fe2+(aq) + 2H2(g)
D) Fe(l) + 2H2O(l) → Fe2+(aq) + 2OH- (aq) + 2H2(g)
E) Fe(l) + 2H2O(l) → Fe2+(aq) + 2OH- (aq) + H2(g)
A) 2Fe(l) + 2H2O(l) → Fe2+(aq) + 3OH-(aq) + 2H2(g)
B) Fe(l) + 2H2O(l) → Fe2+(aq) + H2(g)
C) 2Fe(l) + H2O(l) → Fe2+(aq) + 2H2(g)
D) Fe(l) + 2H2O(l) → Fe2+(aq) + 2OH- (aq) + 2H2(g)
E) Fe(l) + 2H2O(l) → Fe2+(aq) + 2OH- (aq) + H2(g)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
Lead has a greater tendency to be oxidized than chromium.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following elements has the greatest tendency to lose electrons?
A) Magnesium
B) Copper
C) Potassium
D) Manganese
E) Cadmium
A) Magnesium
B) Copper
C) Potassium
D) Manganese
E) Cadmium

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
The _____ is a precipitation that is dramatically more acidic because of human activities and has significant consequences for all the living organisms.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
Acid rain results in the increase of _____ ions in groundwater, which when present in high concentrations, is toxic to plants and affects plant growth.
A) OH-
B) Al3+
C) SO42-
D) NO3−
E) CO32-
A) OH-
B) Al3+
C) SO42-
D) NO3−
E) CO32-

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
The scrubbers in coal-burning power plants use _____ to trap SO2.
A) NO2
B) ZnO
C) CaCO3
D) CaSO4
E) CaO
A) NO2
B) ZnO
C) CaCO3
D) CaSO4
E) CaO

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following reactions shows the formation of a compound that is responsible for the damage of marble buildings and sculptures by acid rain?
A) H2SO3(aq) + Ca(OH)2(s) → CaSO3.2H2O(s)
B) CaO(s) + H2O(l) → Ca(OH)2(s)
C) CaCO3(s) + H2SO4(aq) → CaSO4(s) + H2O(l) + CO2(g)
D) 2N2(g) + 5O2(g) + 2H2O(l) → 4HNO3(aq)
E) 2H2SO3(aq) + O2(g) → 2H2SO4(aq)
A) H2SO3(aq) + Ca(OH)2(s) → CaSO3.2H2O(s)
B) CaO(s) + H2O(l) → Ca(OH)2(s)
C) CaCO3(s) + H2SO4(aq) → CaSO4(s) + H2O(l) + CO2(g)
D) 2N2(g) + 5O2(g) + 2H2O(l) → 4HNO3(aq)
E) 2H2SO3(aq) + O2(g) → 2H2SO4(aq)

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
Acid rain results in the increase in pH of natural waters.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck


