Deck 9: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/152
Play
Full screen (f)
Deck 9: Nuclear Chemistry
1
Identify the product formed by the beta decay of 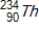 .
.
A)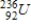
B)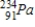
C)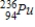
D)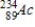
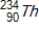 .
.A)
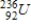
B)
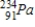
C)
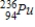
D)
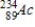
B
2
Which of the following rays are the most penetrating?
A) α rays
B) β rays
C) γ rays
D) β+ rays
A) α rays
B) β rays
C) γ rays
D) β+ rays
C
3
In which of the following elements was radioactivity first observed?
A) sodium
B) radium
C) radon
D) uranium
A) sodium
B) radium
C) radon
D) uranium
D
4
Which of the following rays have the heaviest particles?
A) α rays
B) β rays
C) γ rays
D) β+ rays
A) α rays
B) β rays
C) γ rays
D) β+ rays

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
5
Who among the following scientists discovered radioactivity?
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) E. Rutherford
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) E. Rutherford

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following forms of radiation consists of negatively charged particles?
A) α
B) β
C) γ
D) none of these
A) α
B) β
C) γ
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is the correct order of the frequencies of the electromagnetic spectrum?
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the charge carried by beta (β) particles.
A) 0
B) +2
C) -1
D) -3
A) 0
B) +2
C) -1
D) -3

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
9
What is the speed of light?
A) 3.0 × 108 cm/hour
B) 3.0 × 108 m/s
C) 3.0 × 108 mm/s
D) 3.0 × 108 mm/hour
A) 3.0 × 108 cm/hour
B) 3.0 × 108 m/s
C) 3.0 × 108 mm/s
D) 3.0 × 108 mm/hour

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following equations represents the relationship between the wavelength (λ) and frequency (ν) of electromagnetic radiation?
A) ν =cλ
B) ν =λ/c
C) ν =c/λ
D) ν =1/cλ
A) ν =cλ
B) ν =λ/c
C) ν =c/λ
D) ν =1/cλ

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following equations represents the relationship between the wavelength (λ) and frequency (v) of electromagnetic radiation?
A) λ = cν
B) λ = c/ν
C) λ = ν/c
D) λ = 1/cν
A) λ = cν
B) λ = c/ν
C) λ = ν/c
D) λ = 1/cν

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
12
In which year was Becquerel awarded the Nobel Prize in Physics along with Pierre Curie and Marie Curie?
A) 1910
B) 1905
C) 1900
D) 1903
A) 1910
B) 1905
C) 1900
D) 1903

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following rays consist of helium nuclei?
A) α rays
B) β rays
C) γ rays
D) β+ rays
A) α rays
B) β rays
C) γ rays
D) β+ rays

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following Greek letters is not commonly applied to a form of radioactivity?
A) α
B) β
C) γ
D) δ
A) α
B) β
C) γ
D) δ

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
15
Who among the following scientists discovered X-rays?
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) W. Roentgen
A) H. Becquerel
B) M. Curie
C) A. Einstein
D) W. Roentgen

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following forms of radiation consists of positively charged particles?
A) α
B) β
C) γ
D) none of these
A) α
B) β
C) γ
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a form of electromagnetic radiation?
A) α
B) β
C) γ
D) all of these
A) α
B) β
C) γ
D) all of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following rays are the least penetrating?
A) α rays
B) β rays
C) γ rays
D) β+ rays
A) α rays
B) β rays
C) γ rays
D) β+ rays

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following forms of radiation consists of a hydrogen nucleus?
A) α
B) β
C) γ
D) none of these
A) α
B) β
C) γ
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following forms of radiation is electrically neutral?
A) α
B) β
C) γ
D) none of these
A) α
B) β
C) γ
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is the correct order of the wavelengths of the electromagnetic spectrum?
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared
A) radio > infrared > ultraviolet
B) radio > ultraviolet > infrared
C) ultraviolet > infrared > radio
D) ultraviolet > radio > infrared

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following symbols represents a positron?
A) β−
B)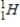
C) β+
D) λ
A) β−
B)
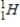
C) β+
D) λ

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements is true of electromagnetic radiation?
A) All electromagnetic radiations have equal energy.
B) As the wavelength of radiation increases, the energy of the radiation decreases.
C) As the wavelength of radiation increases, the energy of the radiation increases.
D) None of these statements are true.
A) All electromagnetic radiations have equal energy.
B) As the wavelength of radiation increases, the energy of the radiation decreases.
C) As the wavelength of radiation increases, the energy of the radiation increases.
D) None of these statements are true.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement is true of the wavelength of an electromagnetic radiation?
A) It refers to the number of wave crests that pass a given point in one second.
B) It is directly proportional to the frequency of the electromagnetic radiation.
C) It is directly proportional to the speed of light in vacuum.
D) It refers to the distance between two successive wave crests in an electromagnetic radiation.
A) It refers to the number of wave crests that pass a given point in one second.
B) It is directly proportional to the frequency of the electromagnetic radiation.
C) It is directly proportional to the speed of light in vacuum.
D) It refers to the distance between two successive wave crests in an electromagnetic radiation.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following is a non-SI unit of energy that is frequently used in nuclear chemistry?
A) the joule
B) the electron volt
C) the kilogram
D) the ampere
A) the joule
B) the electron volt
C) the kilogram
D) the ampere

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the product formed when 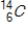 emits a β particle.
emits a β particle.
A)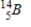
B)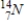
C)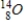
D)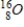
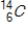 emits a β particle.
emits a β particle.A)
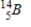
B)
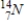
C)
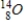
D)
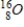

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following Greek letters is used to represent wavelength?
A) ν
B) θ
C) γ
D) λ
A) ν
B) θ
C) γ
D) λ

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements is true of artificially created isotopes?
A) All artificially created isotopes are stable.
B) All artificially created isotopes are unstable.
C) Most artificially created isotopes are stable.
D) Most artificially created isotopes are unstable.
A) All artificially created isotopes are stable.
B) All artificially created isotopes are unstable.
C) Most artificially created isotopes are stable.
D) Most artificially created isotopes are unstable.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following radioactive emissions leads to transmutation in radioactive isotopes?
A) α emission
B) γ emission
C) both a emission and y emission
D) none of these
A) α emission
B) γ emission
C) both a emission and y emission
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
30
Which part of the atom is radioactivity associated with?
A) the core electrons
B) the entire atom
C) the nucleus
D) the valence electrons
A) the core electrons
B) the entire atom
C) the nucleus
D) the valence electrons

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following terms refers to a packet of electromagnetic radiation?
A) proton
B) electron
C) neutron
D) photon
A) proton
B) electron
C) neutron
D) photon

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following radioactive emissions leads to transmutation in radioactive isotopes?
A) β emission
B) γ emission
C) both of these
D) none of these
A) β emission
B) γ emission
C) both of these
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
33
What happens to the mass number of an element when an α particle is emitted from its nucleus?
A) The mass number increases.
B) The mass number decreases.
C) The mass number does not change.
A) The mass number increases.
B) The mass number decreases.
C) The mass number does not change.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
34
Which statement is true of the frequency of an electromagnetic radiation?
A) It is directly proportional to the speed of light in vacuum.
B) It refers to the distance between two successive wave crests in an electromagnetic radiation.
C) It refers to the number of wave crests that pass a given point in one second.
D) The lower the frequency of a wave, the shorter the wavelength.
A) It is directly proportional to the speed of light in vacuum.
B) It refers to the distance between two successive wave crests in an electromagnetic radiation.
C) It refers to the number of wave crests that pass a given point in one second.
D) The lower the frequency of a wave, the shorter the wavelength.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following hydrogen isotopes is radioactive?
A)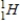
B)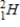
C)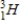
D) none of these
A)
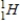
B)
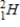
C)
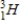
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
36
What happens to the atomic number of an element when an α particle is emitted from its nucleus?
A) The atomic number increases.
B) The atomic number decreases.
C) The atomic number does not change.
A) The atomic number increases.
B) The atomic number decreases.
C) The atomic number does not change.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following radioactive emissions does not lead to transmutation in radioactive isotopes?
A) α emission
B) β emission
C) γ emission
D) None of these radioactive emissions lead to transmutation in radioactive isotopes.
A) α emission
B) β emission
C) γ emission
D) None of these radioactive emissions lead to transmutation in radioactive isotopes.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
38
Identify the product formed when  emits a β particle.
emits a β particle.
A)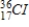
B)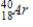
C)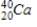
D)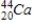
 emits a β particle.
emits a β particle.A)
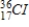
B)
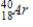
C)
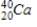
D)
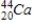

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following Greek letters is used to represent frequency?
A) δ
B) γ
C) ν
D) λ
A) δ
B) γ
C) ν
D) λ

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following statements is true of electromagnetic radiation?
A) All electromagnetic radiations have equal energy.
B) As the frequency of radiation increases, the energy of the radiation decreases.
C) As the frequency of radiation increases, the energy of the radiation increases.
D) None of these statements are true.
A) All electromagnetic radiations have equal energy.
B) As the frequency of radiation increases, the energy of the radiation decreases.
C) As the frequency of radiation increases, the energy of the radiation increases.
D) None of these statements are true.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
41
Identify the element formed when 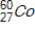 emits γ radiation.
emits γ radiation.
A)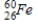
B)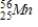
C)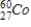
D)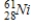
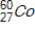 emits γ radiation.
emits γ radiation.A)
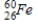
B)
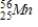
C)
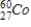
D)
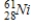

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
42
Identify the element formed when 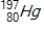 emits γ radiation.
emits γ radiation.
A)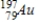
B)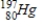
C)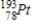
D)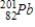
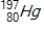 emits γ radiation.
emits γ radiation.A)
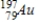
B)
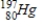
C)
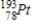
D)
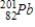

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
43
Identify the symbol used to represent a positron.
A)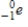
B)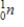
C)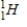
D)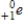
A)
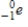
B)
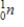
C)
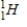
D)
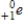

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
44
A radioisotope with mass number A emits a positron. Identify the mass number of the radioisotope formed by the positron emission.
A) A − 2
B) A − 1
C) A
D) A + 1
A) A − 2
B) A − 1
C) A
D) A + 1

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following radioisotopes is formed when 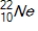 emits a positron?
emits a positron?
A)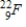
B)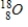
C)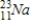
D)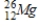
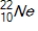 emits a positron?
emits a positron?A)
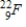
B)
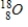
C)
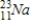
D)
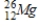

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
46
Polonium-210 is an α emitter. Identify the element formed when 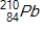 emits an α particle.
emits an α particle.
A)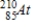
B)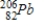
C)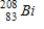
D)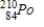
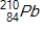 emits an α particle.
emits an α particle.A)
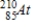
B)
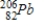
C)
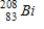
D)
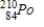

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following isotopes undergoes positron decay to form 24Cr?
A)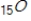
B)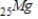
C)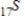
D)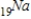
A)
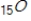
B)
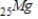
C)
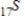
D)
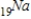

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
48
Identify the element formed when carbon undergoes beta decay.
A) sodium
B) oxygen
C) argon
D) nitrogen
A) sodium
B) oxygen
C) argon
D) nitrogen

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
49
Tritium 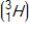 is a β emitter. Identify the product formed when
is a β emitter. Identify the product formed when 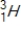 emits a β particle.
emits a β particle.
A)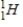
B)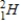
C)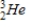
D)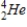
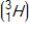 is a β emitter. Identify the product formed when
is a β emitter. Identify the product formed when 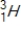 emits a β particle.
emits a β particle.A)
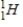
B)
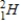
C)
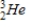
D)
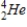

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
50
A radioisotope with atomic number Z emits a positron. Identify the atomic number of the radioisotope formed by the positron emission.
A) Z − 2
B) Z − 1
C) Z + 1
D) Z + 2
A) Z − 2
B) Z − 1
C) Z + 1
D) Z + 2

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the element formed when 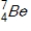 decays by electron capture.
decays by electron capture.
A)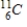
B)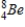
C)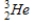
D)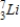
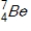 decays by electron capture.
decays by electron capture.A)
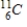
B)
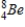
C)
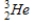
D)
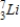

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
52
Arsenic-74 is a positron emitter. Identify the element formed when 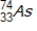 emits a positron.
emits a positron.
A)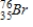
B)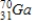
C)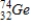
D)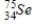
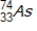 emits a positron.
emits a positron.A)
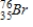
B)
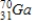
C)
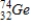
D)
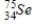

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
53
A radioisotope with atomic number Z emits an α particle. Identify the atomic number of the radioisotope formed by the α emission.
A) Z − 2
B) Z + 4
C) Z − 4
D) Z + 2
A) Z − 2
B) Z + 4
C) Z − 4
D) Z + 2

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
54
Thorium-223 is an α emitter. Identify the element formed when 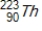 emits an α particle?
emits an α particle?
A)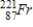
B)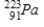
C)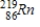
D)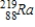
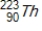 emits an α particle?
emits an α particle?A)
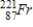
B)
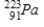
C)
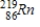
D)
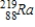

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following affects the half-life of a radioactive sample?
A) the age of the sample
B) the size of the sample
C) the temperature of the sample
D) none of these
A) the age of the sample
B) the size of the sample
C) the temperature of the sample
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
56
Identify the element formed when 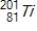 decays by electron capture.
decays by electron capture.
A)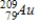
B)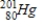
C)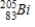
D)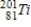
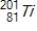 decays by electron capture.
decays by electron capture.A)
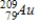
B)
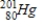
C)
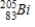
D)
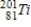

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
57
Identify the element formed when 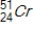 decays by electron capture.
decays by electron capture.
A)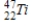
B)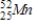
C)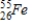
D)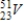
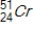 decays by electron capture.
decays by electron capture.A)
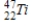
B)
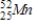
C)
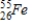
D)
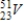

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the product formed when phosphorus-32 undergoes beta decay.
A)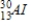
B)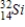
C)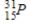
D)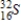
A)
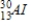
B)
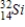
C)
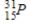
D)
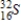

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
59
A radioisotope with atomic number Z undergoes electron capture. Identify the atomic number of the radioisotope formed by the electron capture.
A) Z − 2
B) Z − 1
C) Z + 1
D) Z + 2
A) Z − 2
B) Z − 1
C) Z + 1
D) Z + 2

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
60
A radioisotope with mass number A emits an α particle. Identify the mass number of the radioisotope formed by the α emission.
A) A − 4
B) A − 2
C) A
D) A + 2
A) A − 4
B) A − 2
C) A
D) A + 2

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is true of a radioisotope undergoing beta emission?
A) Its mass number decreases by four units, and its atomic number decreases by two units.
B) Its mass number decreases by one unit, but the atomic number remains the same.
C) Its mass number remains the same, but its atomic number decreases by one unit.
D) Its mass number remains the same, but its atomic number increases by one unit.
A) Its mass number decreases by four units, and its atomic number decreases by two units.
B) Its mass number decreases by one unit, but the atomic number remains the same.
C) Its mass number remains the same, but its atomic number decreases by one unit.
D) Its mass number remains the same, but its atomic number increases by one unit.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following isotopes can be used by geologists to date rocks?
A) C-14
B) I-131
C) Sr-90
D) U-238
A) C-14
B) I-131
C) Sr-90
D) U-238

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
63
Strontium-90 is a dangerous isotope because strontium is chemically similar to calcium and is easily incorporated into bones. Sr-90 has a half-life of 28.1 years. If a person ingests 100 mg of Sr-90, identify the amount that is left in the person's body after 56.2 years.
A) 75.5 mg
B) 50 mg
C) 25 mg
D) 12.5 mg
A) 75.5 mg
B) 50 mg
C) 25 mg
D) 12.5 mg

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
64
Barium-122 has a half-life of 2 minutes. If 10.0 g of Ba-122 is produced in a nuclear reactor, identify the quantity of Ba-122 that will remain 10 minutes after production ceases.
A) 2.50 g
B) 1.25 g
C) 0.625 g
D) 0.313 g
A) 2.50 g
B) 1.25 g
C) 0.625 g
D) 0.313 g

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is the SI unit of radiation activity?
A) the becquerel
B) the curie
C) the millicurie
D) the roentgen
A) the becquerel
B) the curie
C) the millicurie
D) the roentgen

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
66
Identify a radiation detector that contains a phosphor.
A) a Geiger-Müller counter
B) a proportional counter
C) a scintillation counter
D) all of these
A) a Geiger-Müller counter
B) a proportional counter
C) a scintillation counter
D) all of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
67
Identify the percentage of a radioactive sample that remains after four half-lives.
A) 25.0%
B) 12.5%
C) 6.25%
D) 3.13%
A) 25.0%
B) 12.5%
C) 6.25%
D) 3.13%

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
68
Krypton-85 has a half-life of 10 years. Identify the percentage of Kr-85 produced from the Chernobyl accident in 1986 that still remains radioactive in 2010.
A) 25 %
B) 12 %
C) 6 %
D) 3 %
A) 25 %
B) 12 %
C) 6 %
D) 3 %

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following statements is true of a radioisotope undergoing electron capture?
A) A neutron is converted to a proton and an electron.
B) The radioisotope does not undergo transmutation and only the energy changes.
C) An electron reacts with a proton to form a neutron.
D) An electron is absorbed into the nucleus, and an alpha particle is emitted from the radioisotope.
A) A neutron is converted to a proton and an electron.
B) The radioisotope does not undergo transmutation and only the energy changes.
C) An electron reacts with a proton to form a neutron.
D) An electron is absorbed into the nucleus, and an alpha particle is emitted from the radioisotope.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
70
Identify a radiation detector that contains a gas.
A) a Geiger-Müller counter
B) a scintillation counter
C) both a Geiger-Muller counter and a scintillation counter
D) none of these
A) a Geiger-Müller counter
B) a scintillation counter
C) both a Geiger-Muller counter and a scintillation counter
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
71
The half-life of iodine-131 is 8 days. If a medical treatment involves a dose of 400 mg of iodine-131, how much of the isotope remains after 32 days?
A) 200 mg
B) 100 mg
C) 50 mg
D) 25 mg
A) 200 mg
B) 100 mg
C) 50 mg
D) 25 mg

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following particles is emitted during alpha decay?
A)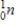
B)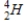
C)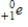
D)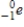
A)
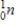
B)
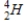
C)
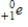
D)
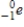

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
73
What particle is emitted from an element when it undergoes beta decay?
A) γ
B)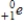
C)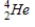
D)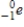
A) γ
B)
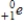
C)
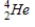
D)
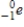

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
74
Which property of a radioactive element is exploited to detect radioactivity?
A) its charge
B) its ionizing ability
C) its mass
D) all of these
A) its charge
B) its ionizing ability
C) its mass
D) all of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
75
Identify the percentage of a radioactive sample that remains after five half-lives.
A) 6.25%
B) 3.13%
C) 1.56%
D) 0.781%
A) 6.25%
B) 3.13%
C) 1.56%
D) 0.781%

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following physical measurements helps characterize ionizing radiation?
A) the energy of emitted particles
B) the intensity of ionizing radiation
C) the energy of emitted particles and the intensity of ionizing radiation
D) none of these
A) the energy of emitted particles
B) the intensity of ionizing radiation
C) the energy of emitted particles and the intensity of ionizing radiation
D) none of these

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following correctly relates the becquerel to the curie?
A) 3.7 × 1010 Bq = 1 Ci
B) 3.7 × 107 Bq = 1 Ci
C) 2.7 × 10-8 Bq = 1 Ci
D) 2.7 × 10-11 Bq = 1 Ci
A) 3.7 × 1010 Bq = 1 Ci
B) 3.7 × 107 Bq = 1 Ci
C) 2.7 × 10-8 Bq = 1 Ci
D) 2.7 × 10-11 Bq = 1 Ci

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
78
The half-life of iodine-131 is 8 days. If a medical treatment involves a dose of 100. mg of iodine-131, how much of the isotope remains after 16 days?
A) 12.5 mg
B) 25 mg
C) 50.5 mg
D) 75 mg
A) 12.5 mg
B) 25 mg
C) 50.5 mg
D) 75 mg

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
79
Which type of radiation emitted by C-14 has a half-life of 5730 years?
A) beta radiation
B) alpha radiation
C) gamma radiation
D) positron radiation
A) beta radiation
B) alpha radiation
C) gamma radiation
D) positron radiation

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following statements is true of a radioisotope that undergoes electron capture?
A) Its mass number decreases by four units, and its atomic number decreases by two units.
B) Its mass number remains the same, but its atomic number increases by one unit.
C) Its mass number remains the same, but its atomic number decreases by one unit.
D) Its mass number and atomic number remain the same, and only its energy changes.
A) Its mass number decreases by four units, and its atomic number decreases by two units.
B) Its mass number remains the same, but its atomic number increases by one unit.
C) Its mass number remains the same, but its atomic number decreases by one unit.
D) Its mass number and atomic number remain the same, and only its energy changes.

Unlock Deck
Unlock for access to all 152 flashcards in this deck.
Unlock Deck
k this deck


