Deck 18: Carboxylic Anhydrides, Esters, and Amides
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/117
Play
Full screen (f)
Deck 18: Carboxylic Anhydrides, Esters, and Amides
1
Which of the following compounds is a cyclic ester?
A) 4-butanolactam
B) 2-propylactane
C) 4-butanolactone
D) 2-propylactam
A) 4-butanolactam
B) 2-propylactane
C) 4-butanolactone
D) 2-propylactam
C
2
Which of the following carboxylic derivatives are rarely found in nature?
A) amides
B) anhydrides
C) esters
D) none of these
A) amides
B) anhydrides
C) esters
D) none of these
B
3
Which of the following compounds is a symmetric anhydride?
A)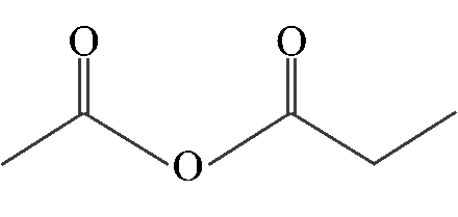
B)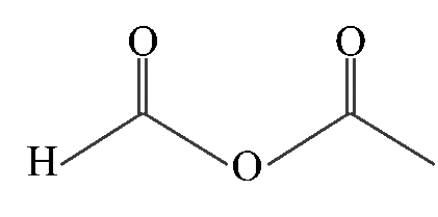
C)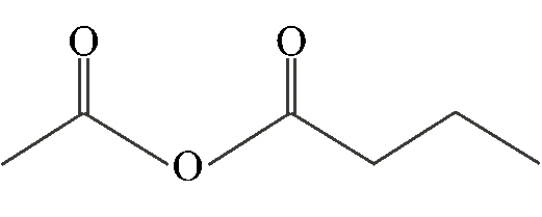
D)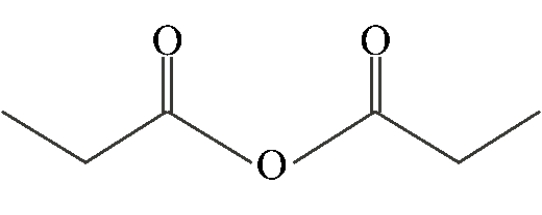
A)

B)

C)

D)

D
4
Which of the following occurs during the formation of an amide?
A) A carboxylic acid loses its -OH group and an ammonia or an amine loses its -H atom.
B) A carboxylic acid loses its -OH group and an alcohol loses its -H atom.
C) An alcohol loses its -OH group and an ammonia or an amine loses its -H atom.
D) None of these are correct.
A) A carboxylic acid loses its -OH group and an ammonia or an amine loses its -H atom.
B) A carboxylic acid loses its -OH group and an alcohol loses its -H atom.
C) An alcohol loses its -OH group and an ammonia or an amine loses its -H atom.
D) None of these are correct.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the IUPAC name of the given compound. 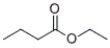
A) propyl ethanoate
B) butyl ethanoate
C) ethyl propanoate
D) ethyl butanoate

A) propyl ethanoate
B) butyl ethanoate
C) ethyl propanoate
D) ethyl butanoate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds has β-lactam ring in its chemical structure?
A) pyrethrin
B) aspirin
C) amoxicillin
D) ibuprofen
A) pyrethrin
B) aspirin
C) amoxicillin
D) ibuprofen

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
7
What is the minimum number of oxygen atoms present in an ester?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following best describes the functional group of anhydrides?
A) two carbonyl groups bonded directly to each other
B) two carboxyl groups bonded directly to each other
C) two carbonyl groups bonded to the same oxygen atom
D) two carboxyl groups bonded to the same oxygen atom
A) two carbonyl groups bonded directly to each other
B) two carboxyl groups bonded directly to each other
C) two carbonyl groups bonded to the same oxygen atom
D) two carboxyl groups bonded to the same oxygen atom

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name of the following compound? 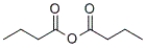
A) butyl butanoate
B) propyl butanoate
C) propanoic anhydride
D) butanoic anhydride

A) butyl butanoate
B) propyl butanoate
C) propanoic anhydride
D) butanoic anhydride

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following are anhydrides? 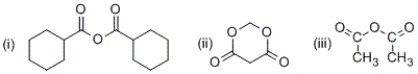
A) only (i) and (ii)
B) only (i) and (iii)
C) only (i) and (iii)
D) (i), (ii) and (iii)

A) only (i) and (ii)
B) only (i) and (iii)
C) only (i) and (iii)
D) (i), (ii) and (iii)

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following occurs during an esterification reaction?
A) An alcohol loses its -OH group and a carboxylic acid loses its -H atom.
B) A carboxylic acid loses its -OH group and an alcohol loses its -H atom.
C) A carboxylic acid loses its -OH group and an amine loses its -H atom.
D) A carboxylic acid loses its double-bonded oxygen and an amine loses two hydrogens.
A) An alcohol loses its -OH group and a carboxylic acid loses its -H atom.
B) A carboxylic acid loses its -OH group and an alcohol loses its -H atom.
C) A carboxylic acid loses its -OH group and an amine loses its -H atom.
D) A carboxylic acid loses its double-bonded oxygen and an amine loses two hydrogens.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
12
Identify a mixed anhydride.
A)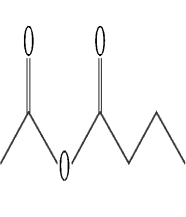
B)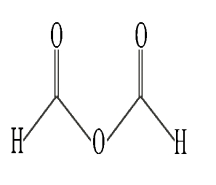
C)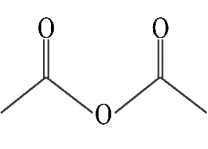
D)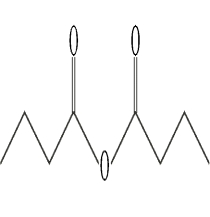
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
13
What is the minimum number of carbon atoms present in an ester?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
14
A chemist treats salicylic acid with acetic anhydride to produce a drug. Identify the drug formed by the reaction.
A) naproxen
B) penicillin G
C) salicin
D) aspirin
A) naproxen
B) penicillin G
C) salicin
D) aspirin

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
15
How many atoms are present in the smallest anhydride?
A) 5
B) 7
C) 9
D) 11
A) 5
B) 7
C) 9
D) 11

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
16
What is the minimum number of oxygen atoms present in an anhydride?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
17
What is the IUPAC name of the following compound? 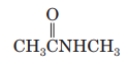
A) ethyl ethanoate
B) methyl ethanoate
C) N-methylacetamide
D) N,N-dimethylacetamide
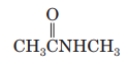
A) ethyl ethanoate
B) methyl ethanoate
C) N-methylacetamide
D) N,N-dimethylacetamide

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
18
Identify a tertiary amide.
A) N,N-dimethylformamide
B) N-methylacetamide
C) acetamide
D) propanamide
A) N,N-dimethylformamide
B) N-methylacetamide
C) acetamide
D) propanamide

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
19
What is the common name of the following compound? 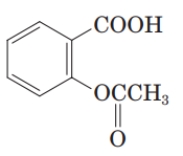
A) salicin
B) aspirin
C) adipic acid
D) barbituric acid

A) salicin
B) aspirin
C) adipic acid
D) barbituric acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
20
What is the minimum number of carbon atoms present in an anhydride?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following pairs of reactants is best suited for the synthesis of aspirin?
A) salicin and acetic acid
B) salicylic acid and acetic acid
C) salicin and acetic anhydride
D) salicylic acid and acetic anhydride
A) salicin and acetic acid
B) salicylic acid and acetic acid
C) salicin and acetic anhydride
D) salicylic acid and acetic anhydride

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
22
Which pair of reactants forms ethyl butanoate during an esterification reaction?
A) formic acid and 1-butanol
B) butanoic acid and ethanol
C) acetic acid and methanol
D) ethanoic acid and 2-butanol
A) formic acid and 1-butanol
B) butanoic acid and ethanol
C) acetic acid and methanol
D) ethanoic acid and 2-butanol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
23
Which ester is formed by the reaction of CH3CH2COOH with CH3CH2OH?
A) ethyl acetate
B) ethyl propanoate
C) propyl acetate
D) propyl acetate
A) ethyl acetate
B) ethyl propanoate
C) propyl acetate
D) propyl acetate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following statements is true of the structural composition of β-lactam ring?
A) It consists of a seven-membered, aromatic, heterocyclic ester.
B) It consists of a five-membered, aliphatic, heterocyclic ester.
C) It consists of a seven-membered, aromatic, heterocyclic amide.
D) It consists of a four-membered, aliphatic, heterocyclic amide.
A) It consists of a seven-membered, aromatic, heterocyclic ester.
B) It consists of a five-membered, aliphatic, heterocyclic ester.
C) It consists of a seven-membered, aromatic, heterocyclic amide.
D) It consists of a four-membered, aliphatic, heterocyclic amide.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following products is formed when acetic acid reacts with propanol?
A) ethyl acetate
B) ethyl propanoate
C) propyl acetate
D) none of these
A) ethyl acetate
B) ethyl propanoate
C) propyl acetate
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the product formed by the transesterification of the given ester with ethanol. 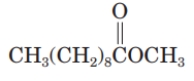
A) butanoic acid and 1-butanol
B) decanoic acid and ethanol
C) methyl butanoate and 1-butanol
D) ethyl decanoate and methanol
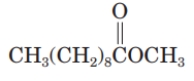
A) butanoic acid and 1-butanol
B) decanoic acid and ethanol
C) methyl butanoate and 1-butanol
D) ethyl decanoate and methanol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
27
Identify the key enzyme involved in the conversion of arachidonic acid to prostaglandins.
A) esterase
B) β-lactamase
C) cyclooxygenase
D) polymerase
A) esterase
B) β-lactamase
C) cyclooxygenase
D) polymerase

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
28
What is the IUPAC name of a compound with the common name adipamide?
A) hexanediamide
B) benzamide
C) butanamide
D) pentanamide
A) hexanediamide
B) benzamide
C) butanamide
D) pentanamide

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
29
What is the IUPAC name of the following compound? 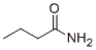
A) aminopropanoate
B) propanamide
C) butanoylamine
D) butanamide

A) aminopropanoate
B) propanamide
C) butanoylamine
D) butanamide

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
30
Identify a commonly used catalyst in a Fischer esterification reaction.
A) acetic acid
B) dilute hydrochloric acid
C) dilute carbonic acid
D) concentrated sulfuric acid
A) acetic acid
B) dilute hydrochloric acid
C) dilute carbonic acid
D) concentrated sulfuric acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following types of compounds are cyclic amides?
A) lactams
B) lactanes
C) lactines
D) lactones
A) lactams
B) lactanes
C) lactines
D) lactones

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
32
Salicin is hydrolyzed in an aqueous acidic solution to form an intermediate. Then, the intermediate is oxidized to form a product. Identify the product formed at the end of the reaction.
A) adipic acid
B) ibuprofen
C) salicylic acid
D) salicylate
A) adipic acid
B) ibuprofen
C) salicylic acid
D) salicylate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
33
Which pair of reactants forms butyl formate during an esterification reaction?
A) formic acid and 1-butanol
B) formamide and 1-butanol
C) butanoic acid and ethanol
D) butanamide and methanol
A) formic acid and 1-butanol
B) formamide and 1-butanol
C) butanoic acid and ethanol
D) butanamide and methanol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
34
Which enantiomer of ibuprofen inhibits cyclooxygenase (COX) activity?
A) (R)-ibuprofen
B) (S)-ibuprofen
C) both (R)-ibuprofen and (S)-ibuprofen
D) none of these
A) (R)-ibuprofen
B) (S)-ibuprofen
C) both (R)-ibuprofen and (S)-ibuprofen
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
35
What is the common name of the given compound? 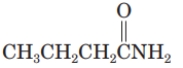
A) adipamide
B) benzamide
C) butyramide
D) propanamide
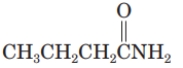
A) adipamide
B) benzamide
C) butyramide
D) propanamide

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements is true of natural pyrethrins?
A) They are esters of chrysanthemic acid.
B) They are amides of permethrin.
C) They are esters of permethrin.
D) They are amides of chrysanthemic acid.
A) They are esters of chrysanthemic acid.
B) They are amides of permethrin.
C) They are esters of permethrin.
D) They are amides of chrysanthemic acid.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
37
Identify the functional group present in natural pyrethrins.
A) amide
B) anhydride
C) ester
D) none of these
A) amide
B) anhydride
C) ester
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
38
For which of the following organisms are pyrethrins poisonous?
A) insects
B) mammals
C) plants
D) all of these
A) insects
B) mammals
C) plants
D) all of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
39
What is the IUPAC name of the following compound? 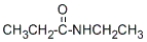
A) ethylpropanamide
B) N-ethylpropanamide
C) N-ethylethanamide
D) pentanamide
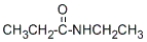
A) ethylpropanamide
B) N-ethylpropanamide
C) N-ethylethanamide
D) pentanamide

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the IUPAC name of the following compound. 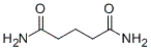
A) diamino pentanoate
B) pentanediamine
C) pentanediamide
D) diamidopropane

A) diamino pentanoate
B) pentanediamine
C) pentanediamide
D) diamidopropane

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following products is formed when an ammonium carboxylate salt is heated?
A) an amide
B) an amino acid
C) an amide and an amino acid
D) none of these
A) an amide
B) an amino acid
C) an amide and an amino acid
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
42
Which statement about the acid-catalyzed hydrolysis of esters is not true?
A) Using one equivalent of water gives a high yield of products.
B) Using excess water gives a high yield of products.
C) Removing water from the equilibrium mixture gives a high yield of products.
D) All these statements are false.
A) Using one equivalent of water gives a high yield of products.
B) Using excess water gives a high yield of products.
C) Removing water from the equilibrium mixture gives a high yield of products.
D) All these statements are false.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following compounds is formed by the reaction of formic anhydride with ammonia?
A) formamide
B) N-methylformamide
C) N,N-dimethylformamide
D) acetamide
A) formamide
B) N-methylformamide
C) N,N-dimethylformamide
D) acetamide

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the products is always formed during the hydrolysis of esters, regardless of whether the hydrolysis is carried out in an acidic or a basic solution?
A) an aldehyde
B) a carboxylic acid
C) a salt
D) none of these
A) an aldehyde
B) a carboxylic acid
C) a salt
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following pairs of products is formed when ethyl benzoate is hydrolyzed in an acidic solution?
A) benzoic acid and an ethanoate salt
B) ethanol and a benzoate salt
C) benzoic acid and ethanol
D) benzoic anhydride and methanol
A) benzoic acid and an ethanoate salt
B) ethanol and a benzoate salt
C) benzoic acid and ethanol
D) benzoic anhydride and methanol

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
46
Identify the product formed when propanoic anhydride is treated with diethylamine.
A) propanamide
B) N-ethylpropanamide
C) N,N-diethylpropanamide
D) ethyl propanoate
A) propanamide
B) N-ethylpropanamide
C) N,N-diethylpropanamide
D) ethyl propanoate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following by-products is obtained when an amine and an anhydride react to form an amide?
A) an alcohol
B) a carboxylic acid
C) water
D) an ester
A) an alcohol
B) a carboxylic acid
C) water
D) an ester

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements is true of ester hydrolysis?
A) It occurs rapidly even in cold water.
B) It occurs rapidly at room temperature.
C) It occurs rapidly in boiling water.
D) None of these statements are correct.
A) It occurs rapidly even in cold water.
B) It occurs rapidly at room temperature.
C) It occurs rapidly in boiling water.
D) None of these statements are correct.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following compounds is formed by the reaction of formic anhydride with methylamine?
A) formamide
B) N-methylformamide
C) N,N-dimethylformamide
D) a mixture of two or more of these compounds
A) formamide
B) N-methylformamide
C) N,N-dimethylformamide
D) a mixture of two or more of these compounds

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements is true of a reaction between a low-molecular-weight anhydride and water?
A) The anhydride physically absorbs the water, but no chemical reaction occurs.
B) The anhydride reacts slowly with water to produce two molecules of carboxylic acid.
C) The anhydride reacts readily with water to produce two molecules of carboxylic acid.
D) Whether or not the anhydride reacts with water depends on its molecular weight.
A) The anhydride physically absorbs the water, but no chemical reaction occurs.
B) The anhydride reacts slowly with water to produce two molecules of carboxylic acid.
C) The anhydride reacts readily with water to produce two molecules of carboxylic acid.
D) Whether or not the anhydride reacts with water depends on its molecular weight.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the compound obtained when an amine reacts with a carboxylic acid at room temperature.
A) an amide
B) an amino acid
C) an ammonium carboxylate salt
D) none of these
A) an amide
B) an amino acid
C) an ammonium carboxylate salt
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following statements is true of ester hydrolysis?
A) Both an acid and a base can act as the true catalysts of the reaction.
B) Neither an acid nor a base is the true catalyst of the reaction.
C) An acid is a true catalyst, but a base is a reagent and must be present in the appropriate stoichiometric amount.
D) A base is a true catalyst, but an acid is a reagent and must be present in the appropriate stoichiometric amount.
A) Both an acid and a base can act as the true catalysts of the reaction.
B) Neither an acid nor a base is the true catalyst of the reaction.
C) An acid is a true catalyst, but a base is a reagent and must be present in the appropriate stoichiometric amount.
D) A base is a true catalyst, but an acid is a reagent and must be present in the appropriate stoichiometric amount.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following can be used to mediate the hydrolysis of esters?
A) aqueous sulfuric acid
B) aqueous sodium hydroxide
C) aqueous sulfuric acid and aqueous sodium hydroxide
D) none of these
A) aqueous sulfuric acid
B) aqueous sodium hydroxide
C) aqueous sulfuric acid and aqueous sodium hydroxide
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following compounds would react together at room temperature to form the given compound? 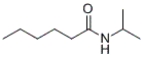
A) hexanamide and 2-propanol
B) hexanoic acid and 2-propanamine
C) 2-propanamine and hexanoic anhydride
D) pentanoic acid and 2-propanamine

A) hexanamide and 2-propanol
B) hexanoic acid and 2-propanamine
C) 2-propanamine and hexanoic anhydride
D) pentanoic acid and 2-propanamine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the products is always formed during the hydrolysis of esters, regardless of whether the hydrolysis is carried out in an acidic or a basic solution?
A) an alcohol
B) carboxylic acid
C) a salt
D) none of these
A) an alcohol
B) carboxylic acid
C) a salt
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
56
Identify the products formed by the acid-catalyzed hydrolysis of methyl propanoate.
A) methanol and propanoic acid
B) methanol and a propanoate salt
C) propanol and formic acid
D) propanol and a formate salt
A) methanol and propanoic acid
B) methanol and a propanoate salt
C) propanol and formic acid
D) propanol and a formate salt

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following pairs of products is formed by the acid-catalyzed hydrolysis of ethyl formate?
A) ethanol and a formate salt
B) ethanol and formic acid
C) methanol and an ethanoate salt
D) methanol and ethanoic acid
A) ethanol and a formate salt
B) ethanol and formic acid
C) methanol and an ethanoate salt
D) methanol and ethanoic acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
58
Which combination of reactants is commonly used to synthesize amides at room temperature?
A) an amine and an anhydride
B) an amine and an alcohol
C) an amine and a carboxylic acid
D) none of these
A) an amine and an anhydride
B) an amine and an alcohol
C) an amine and a carboxylic acid
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
59
Which pair of products is formed when ethyl butanoate is hydrolyzed in a basic solution?
A) ethanol and a butanoate salt
B) ethanol and propanoic acid
C) butanol and an acetate salt
D) propanol and acetic acid
A) ethanol and a butanoate salt
B) ethanol and propanoic acid
C) butanol and an acetate salt
D) propanol and acetic acid

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
60
Predict the products formed in the given reaction. 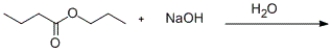
A) 1-propanol and butanoic acid
B) 1-propanol and sodium butanoate
C) 1-butanol and propanoic acid
D) 1-butanol and sodium propanoate
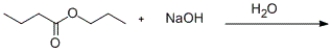
A) 1-propanol and butanoic acid
B) 1-propanol and sodium butanoate
C) 1-butanol and propanoic acid
D) 1-butanol and sodium propanoate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following statements is true regarding carboxylic anhydrides and phosphoric anhydrides?
A) Both carboxylic anhydrides and phosphoric anhydrides have important biological functions.
B) Neither carboxylic anhydrides nor phosphoric anhydrides have important biological functions.
C) Carboxylic anhydrides are not important in biochemistry, but phosphoric anhydrides are important in biochemistry.
D) Phosphoric anhydrides are not important in biochemistry, but carboxylic anhydrides are important in biochemistry.
A) Both carboxylic anhydrides and phosphoric anhydrides have important biological functions.
B) Neither carboxylic anhydrides nor phosphoric anhydrides have important biological functions.
C) Carboxylic anhydrides are not important in biochemistry, but phosphoric anhydrides are important in biochemistry.
D) Phosphoric anhydrides are not important in biochemistry, but carboxylic anhydrides are important in biochemistry.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following are produced when acetic anhydride reacts with methanol?
A) only propanoic acid
B) only methyl acetate
C) formic acid and methyl acetate
D) acetic acid and methyl acetate
A) only propanoic acid
B) only methyl acetate
C) formic acid and methyl acetate
D) acetic acid and methyl acetate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following compounds is formed when an amide reacts with an amine?
A) a carboxylic acid
B) an ester
C) an imide
D) None, amides do not react with amines.
A) a carboxylic acid
B) an ester
C) an imide
D) None, amides do not react with amines.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
64
Predict the major products of the given reaction. 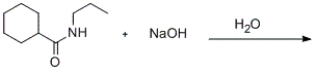
A)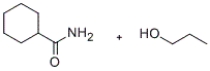
B)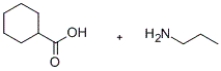
C)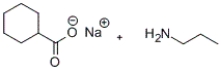
D)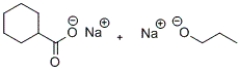

A)

B)
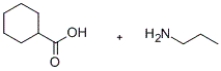
C)

D)


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following gives the correct order of ease of hydrolysis (with easiest to hydrolyze listed first)?
A) anhydrides, esters, amides
B) anhydrides, amides, esters
C) amides, esters, anhydrides
D) amides, anhydrides, esters
A) anhydrides, esters, amides
B) anhydrides, amides, esters
C) amides, esters, anhydrides
D) amides, anhydrides, esters

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following types of compounds is formed when an anhydride reacts with an alcohol?
A) only a carboxylic acid
B) only an ester
C) a carboxylic acid and an ester
D) none of these
A) only a carboxylic acid
B) only an ester
C) a carboxylic acid and an ester
D) none of these

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
67
Identify the products formed when acetamide is hydrolyzed in hydrochloric acid.
A) sodium acetate and acetic acid
B) sodium acetate and ammonia
C) acetic acid and ammonium chloride
D) acetic acid and ammonia
A) sodium acetate and acetic acid
B) sodium acetate and ammonia
C) acetic acid and ammonium chloride
D) acetic acid and ammonia

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following statements is true of amide hydrolysis?
A) Both an acid and a base can act as the true catalysts of the reaction.
B) Neither an acid nor a base is the true catalyst of the reaction.
C) An acid is a true catalyst, but a base is a reagent and must be present in the appropriate stoichiometric amount.
D) A base is a true catalyst, but an acid is a reagent and must be present in the appropriate stoichiometric amount.
A) Both an acid and a base can act as the true catalysts of the reaction.
B) Neither an acid nor a base is the true catalyst of the reaction.
C) An acid is a true catalyst, but a base is a reagent and must be present in the appropriate stoichiometric amount.
D) A base is a true catalyst, but an acid is a reagent and must be present in the appropriate stoichiometric amount.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
69
Predict the major products of the given reaction. 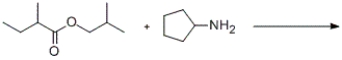
A)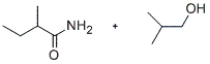
B)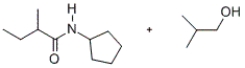
C)
D)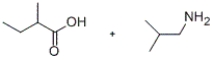

A)
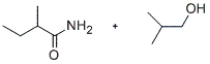
B)

C)

D)


Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
70
Which pair of products is formed when formic anhydride reacts with ethanol?
A) acetic acid and ethyl formate
B) acetic acid and methyl formate
C) formic acid and methyl formate
D) formic acid and ethyl formate
A) acetic acid and ethyl formate
B) acetic acid and methyl formate
C) formic acid and methyl formate
D) formic acid and ethyl formate

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following statements is true of amide hydrolysis?
A) The reaction is reversible in either an acidic or a basic solution.
B) The reaction is irreversible in both acidic and basic solutions.
C) The reaction is reversible in an acidic solution but irreversible in a basic solution.
D) The reaction is reversible in a basic solution but irreversible in an acidic solution.
A) The reaction is reversible in either an acidic or a basic solution.
B) The reaction is irreversible in both acidic and basic solutions.
C) The reaction is reversible in an acidic solution but irreversible in a basic solution.
D) The reaction is reversible in a basic solution but irreversible in an acidic solution.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
72
How many moles of neutral organic compounds will be formed when one mole of the given compound is hydrolyzed in an aqueous solution containing an excess of sodium hydroxide? 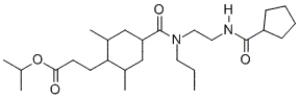
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
73
Which types of amines do not react with esters to form amides?
A) 1°
B) 2°
C) 3°
D) All these types of amines react with esters to form amides.
A) 1°
B) 2°
C) 3°
D) All these types of amines react with esters to form amides.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following are the products of the hydrolysis of an amide in a basic solution?
A) a carboxylic acid and an ammonium salt
B) a carboxylic acid and an amine
C) a carboxylate salt and an ammonium salt
D) a carboxylate salt and an amine
A) a carboxylic acid and an ammonium salt
B) a carboxylic acid and an amine
C) a carboxylate salt and an ammonium salt
D) a carboxylate salt and an amine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following are the products of the hydrolysis of an amide in an acidic solution?
A) a carboxylic acid and an ammonium salt
B) a carboxylic acid and an amine
C) a carboxylate salt and an ammonium salt
D) a carboxylate salt and an amine
A) a carboxylic acid and an ammonium salt
B) a carboxylic acid and an amine
C) a carboxylate salt and an ammonium salt
D) a carboxylate salt and an amine

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following statements is correct about the core ring structure of barbituric acid?
A) It is an aromatic ring that has three carbons and three nitrogens.
B) It is an aromatic ring that has four carbons and two nitrogens.
C) It is an aliphatic ring that has three carbons and three nitrogens.
D) It is an aliphatic ring that has four carbons and two nitrogens.
A) It is an aromatic ring that has three carbons and three nitrogens.
B) It is an aromatic ring that has four carbons and two nitrogens.
C) It is an aliphatic ring that has three carbons and three nitrogens.
D) It is an aliphatic ring that has four carbons and two nitrogens.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following types of amines do not react with anhydrides to form amides?
A) 1° amines
B) 2° amines
C) 3° amines
D) All these types of amines react with anhydrides to form amides.
A) 1° amines
B) 2° amines
C) 3° amines
D) All these types of amines react with anhydrides to form amides.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
78
Identify the products formed when acetamide is hydrolyzed in sodium hydroxide.
A) sodium acetate and acetic acid
B) sodium acetate and ammonia
C) acetic acid and ammonium chloride
D) acetic acid and ammonia
A) sodium acetate and acetic acid
B) sodium acetate and ammonia
C) acetic acid and ammonium chloride
D) acetic acid and ammonia

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following statements is true of amide hydrolysis?
A) Amides do not undergo hydrolysis.
B) Amides are more difficult to hydrolyze than esters.
C) Amides and esters are hydrolyzed under similar conditions.
D) Amides are more easily hydrolyzed than esters.
A) Amides do not undergo hydrolysis.
B) Amides are more difficult to hydrolyze than esters.
C) Amides and esters are hydrolyzed under similar conditions.
D) Amides are more easily hydrolyzed than esters.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck
80
What is the correct stoichiometric ratio for the reactants when anhydrides react with amines to form an amide and an ammonium carboxylate salt?
A) one mole of amine for each mole of anhydride
B) one mole of amine for every two moles of anhydride
C) two moles of amine for every mole of anhydride
D) The ratio will depend on the identity of the amine and the anhydride.
A) one mole of amine for each mole of anhydride
B) one mole of amine for every two moles of anhydride
C) two moles of amine for every mole of anhydride
D) The ratio will depend on the identity of the amine and the anhydride.

Unlock Deck
Unlock for access to all 117 flashcards in this deck.
Unlock Deck
k this deck


