Deck 17: Carboxylic Acids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/115
Play
Full screen (f)
Deck 17: Carboxylic Acids
1
Which of the following is not a real compound?
A) 1-hydroxybutanoic acid
B) 2-hydroxybutanoic acid
C) 3-hydroxybutanoic acid
D) none of these
A) 1-hydroxybutanoic acid
B) 2-hydroxybutanoic acid
C) 3-hydroxybutanoic acid
D) none of these
A
2
Which of the following is a major component of kidney stones?
A) calcium glutarate
B) calcium malate
C) calcium oxalate
D) calcium succinate
A) calcium glutarate
B) calcium malate
C) calcium oxalate
D) calcium succinate
C
3
Which is the correct IUPAC name of the carboxylic acid that contains five carbon atoms?
A) 1-methyl-1-butanoic acid
B) 1,1-dimethylpropanoic acid
C) 1,2-dimethylpropanoic acid
D) 2-methylbutanoic acid
A) 1-methyl-1-butanoic acid
B) 1,1-dimethylpropanoic acid
C) 1,2-dimethylpropanoic acid
D) 2-methylbutanoic acid
D
4
Which of the following is γ-hydroxybutyric acid?
A)
B)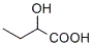
C)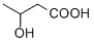
D)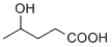
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
5
How many carboxylic acids have the molecular formula C4H8O2?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the correct structure of caproic acid?
A) CH3(CH2)2COOH
B) CH3(CH2)3COOH
C) CH3(CH2)4COOH
D) CH3(CH2)10COOH
A) CH3(CH2)2COOH
B) CH3(CH2)3COOH
C) CH3(CH2)4COOH
D) CH3(CH2)10COOH

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds contain(s) a carboxylic acid functional group? 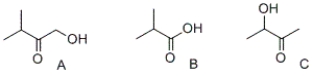
A) A and B only
B) A and C only
C) C only
D) B only

A) A and B only
B) A and C only
C) C only
D) B only

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the nonpolar covalent bond present in ethanoic acid.
A) C-O single bond
B) C-O double bond
C) C-H single bond
D) O-H single bond
A) C-O single bond
B) C-O double bond
C) C-H single bond
D) O-H single bond

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following must be present in a carboxylic acid?
A) a carbonyl group and a hydroxyl group bonded to different carbon atoms
B) a carbonyl group and a hydroxyl group bonded to the same carbon atom
C) two hydroxyl groups bonded to the same carbon atom
D) two carbonyl groups bonded to the same carbon atom
A) a carbonyl group and a hydroxyl group bonded to different carbon atoms
B) a carbonyl group and a hydroxyl group bonded to the same carbon atom
C) two hydroxyl groups bonded to the same carbon atom
D) two carbonyl groups bonded to the same carbon atom

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is an intermediate in the citric acid cycle?
A) glutaric acid
B) oxalic acid
C) succinic acid
D) valeric acid
A) glutaric acid
B) oxalic acid
C) succinic acid
D) valeric acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following acids is a component of the venom injected by stinging ants?
A) butyric acid
B) acetic acid
C) formic acid
D) stearic acid
A) butyric acid
B) acetic acid
C) formic acid
D) stearic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
12
What is the IUPAC name of GABA?
A) 3-amino-2-propenoic acid
B) 4-aminobutanoic acid
C) 4-amino-benzoic acid
D) 4-carboxybutylamine
A) 3-amino-2-propenoic acid
B) 4-aminobutanoic acid
C) 4-amino-benzoic acid
D) 4-carboxybutylamine

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is not a dicarboxylic acid?
A) glutaric acid
B) oxalic acid
C) succinic acid
D) none of these
A) glutaric acid
B) oxalic acid
C) succinic acid
D) none of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
14
What is the IUPAC name of the following carboxylic acid? 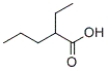
A) 3-carboxyhexane
B) 2-ethylpentanoic acid
C) 4-ethylpentanoic acid
D) 2-propylbutanoic acid

A) 3-carboxyhexane
B) 2-ethylpentanoic acid
C) 4-ethylpentanoic acid
D) 2-propylbutanoic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
15
Which is the correct IUPAC name of the carboxylic acid that contains three carbon atoms?
A) 2-methylpropanoic acid
B) propanoic acid
C) 2-propionic acid
D) ethanedioic acid
A) 2-methylpropanoic acid
B) propanoic acid
C) 2-propionic acid
D) ethanedioic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
16
What is the IUPAC name of the straight chain dicarboxylic acid that contains seven carbon atoms?
A) 1,6-heptanedioic acid
B) heptanedioic acid
C) 1,5-pentanedioic acid
D) pentanedioic acid
A) 1,6-heptanedioic acid
B) heptanedioic acid
C) 1,5-pentanedioic acid
D) pentanedioic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
17
Which is the correct IUPAC name of the carboxylic acid that contains four carbon atoms?
A) butanoic acid
B) isobutyric acid
C) 2-butanoic acid
D) butyric acid
A) butanoic acid
B) isobutyric acid
C) 2-butanoic acid
D) butyric acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
18
Identify the structure of decanoic acid.
A) CH3-(CH2)4-COOH
B) CH3-(CH2)6-COOH
C) CH3-(CH2)8-COOH
D) CH3-(CH2)10-COOH
A) CH3-(CH2)4-COOH
B) CH3-(CH2)6-COOH
C) CH3-(CH2)8-COOH
D) CH3-(CH2)10-COOH

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
19
What is the IUPAC name of the following compound? 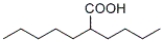
A) 5-carboxydecane
B) 5-pentylhexanoic acid
C) 2-pentylhexanoic acid
D) 2-butylheptanoic acid

A) 5-carboxydecane
B) 5-pentylhexanoic acid
C) 2-pentylhexanoic acid
D) 2-butylheptanoic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
20
What is the IUPAC name of the carboxylic acid that contains three carbon atoms and a terminal amine group?
A) 3-carboxyethylamine
B) 1-amino-3-propanoic acid
C) 3-carboxypropylamine
D) 3-aminopropanoic acid
A) 3-carboxyethylamine
B) 1-amino-3-propanoic acid
C) 3-carboxypropylamine
D) 3-aminopropanoic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
21
Identify the fatty acid denoted by 20:0.
A) lauric acid
B) palmitic acid
C) stearic acid
D) arachidic acid
A) lauric acid
B) palmitic acid
C) stearic acid
D) arachidic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
22
Identify the difference between oxalic acid and malonic acid.
A) Oxalic acid has one carboxylic group, whereas malonic acid has two carboxylic groups.
B) Oxalic acid has two carboxylic groups, whereas malonic acid has one carboxylic group.
C) Oxalic acid has two carbon atoms, whereas malonic acid has three carbon atoms.
D) Oxalic acid has three carbon atoms, whereas malonic acid has two carbon atoms.
A) Oxalic acid has one carboxylic group, whereas malonic acid has two carboxylic groups.
B) Oxalic acid has two carboxylic groups, whereas malonic acid has one carboxylic group.
C) Oxalic acid has two carbon atoms, whereas malonic acid has three carbon atoms.
D) Oxalic acid has three carbon atoms, whereas malonic acid has two carbon atoms.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following acids is the least soluble in water?
A) acetic acid
B) propionic acid
C) capric acid
D) butyric acid
A) acetic acid
B) propionic acid
C) capric acid
D) butyric acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
24
Identify the correct structure of stearic acid.
A) CH3-(CH2)4-COOH
B) CH3-(CH2)8-COOH
C) CH3-(CH2)10-COOH
D) CH3-(CH2)16-COOH
A) CH3-(CH2)4-COOH
B) CH3-(CH2)8-COOH
C) CH3-(CH2)10-COOH
D) CH3-(CH2)16-COOH

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
25
In a sample of a pure carboxylic acid, how many hydrogen bonds are formed between a pair of carboxylic acid molecules?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the reaction that involves the conversion of butanoic acid to propane by heating it to a very high temperature.
A) oxidation
B) esterification
C) decarboxylation
D) hydrogenation
A) oxidation
B) esterification
C) decarboxylation
D) hydrogenation

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following compounds is most soluble in water? 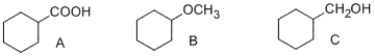
A) A
B) C
C) B
D) none of these

A) A
B) C
C) B
D) none of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
28
Identify the acid that is primarily responsible for the sour taste of pickles and sauerkraut.
A) pentanoic acid
B) citric acid
C) lactic acid
D) butanoic acid
A) pentanoic acid
B) citric acid
C) lactic acid
D) butanoic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following acids is the most soluble in water?
A) caprylic acid
B) caproic acid
C) butyric acid
D) lauric acid
A) caprylic acid
B) caproic acid
C) butyric acid
D) lauric acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is correct about the solubilities of propanoic acid and propanal in water?
A) Water solubility of propanoic acid is significantly less than propanal.
B) Water solubility of propanoic acid is slightly less than propanal.
C) Water solubility of propanoic acid is slightly more than propanal.
D) Water solubility of propanoic acid is significantly more than propanal.
A) Water solubility of propanoic acid is significantly less than propanal.
B) Water solubility of propanoic acid is slightly less than propanal.
C) Water solubility of propanoic acid is slightly more than propanal.
D) Water solubility of propanoic acid is significantly more than propanal.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
31
GABA is the abbreviation for the neurotransmitter γ-aminobutyric acid. Which of the following is the structure of GABA?
A)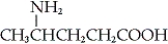
B)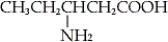
C) NH2CH2CH2CH2COOH
D) none of these
A)
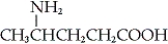
B)
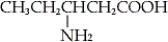
C) NH2CH2CH2CH2COOH
D) none of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following acids is used in the synthesis of the polymer nylon-66?
A) adipic acid
B) palmitic acid
C) stearic acid
D) formic acid
A) adipic acid
B) palmitic acid
C) stearic acid
D) formic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds will have the lowest boiling point?
A) butanoic acid
B) 1-proponol
C) acetic acid
D) propanal
A) butanoic acid
B) 1-proponol
C) acetic acid
D) propanal

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following statements best compares the boiling points of carboxylic acids and other neutral organic compounds of comparable weight?
A) They are significantly lower than the boiling points of the other compounds.
B) They are slightly lower than the boiling points of the other compounds.
C) They are slightly higher than the boiling points of the other compounds.
D) They are significantly higher than the boiling points of the other compounds.
A) They are significantly lower than the boiling points of the other compounds.
B) They are slightly lower than the boiling points of the other compounds.
C) They are slightly higher than the boiling points of the other compounds.
D) They are significantly higher than the boiling points of the other compounds.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following compounds has the highest boiling point?
A) butanoic acid
B) 1-proponol
C) acetic acid
D) propanal
A) butanoic acid
B) 1-proponol
C) acetic acid
D) propanal

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
36
Identify the fatty acid denoted by 18:2.
A) stearic acid
B) palmitoleic acid
C) linoleic acid
D) arachidonic acid
A) stearic acid
B) palmitoleic acid
C) linoleic acid
D) arachidonic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
37
How are fatty acids obtained from animal fats?
A) by hydrolysis
B) by reduction
C) by oxidation
D) by substitution
A) by hydrolysis
B) by reduction
C) by oxidation
D) by substitution

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
38
What is the IUPAC name of the following compound? 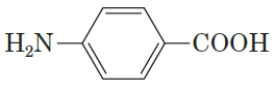
A) 3-carboxyaniline
B) 4-aminobenzoic acid
C) 4-carboxyaniline
D) m-aminobenzoic acid

A) 3-carboxyaniline
B) 4-aminobenzoic acid
C) 4-carboxyaniline
D) m-aminobenzoic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
39
What is the IUPAC name of the following compound? 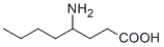
A) 3-aminoheptanoic acid
B) 4-aminooctanoic acid
C) 4-amino-4-butylbutanoic acid
D) γ-aminobutyric acid

A) 3-aminoheptanoic acid
B) 4-aminooctanoic acid
C) 4-amino-4-butylbutanoic acid
D) γ-aminobutyric acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
40
What is the IUPAC name of the following compound? 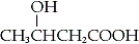
A) 2-hydroxy-4-butanoic acid
B) 3-hydroxybutanoic acid
C) α-hydroxybutanoic acid
D) γ-hydroxybutanoic acid
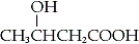
A) 2-hydroxy-4-butanoic acid
B) 3-hydroxybutanoic acid
C) α-hydroxybutanoic acid
D) γ-hydroxybutanoic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following notations corresponds to the fatty acid with the lowest melting point?
A) 18:0
B) 18:1
C) 18:2
D) 18:3
A) 18:0
B) 18:1
C) 18:2
D) 18:3

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following 18 carbon fatty acids will have the highest melting point?
A) oleic acid
B) linoleic acid
C) linolenic acid
D) stearic acid
A) oleic acid
B) linoleic acid
C) linolenic acid
D) stearic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following fatty acids is saturated?
A) lauric acid
B) palmitic acid
C) stearic acid
D) all of these
A) lauric acid
B) palmitic acid
C) stearic acid
D) all of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is true of linolenic acid?
A) It has two double bonds.
B) It has four double bonds.
C) It has five double bonds.
D) It has three double bonds.
A) It has two double bonds.
B) It has four double bonds.
C) It has five double bonds.
D) It has three double bonds.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
45
Why do saturated fatty acids have higher melting points than cis-unsaturated fatty acids of comparable molecular weight.
A) because saturated fatty acids have stronger repulsive forces between their hydrocarbon chains than unsaturated fatty acids do
B) because saturated fatty acids have stronger hydrogen bonds between their hydrocarbon chains than unsaturated fatty acids do
C) because saturated fatty acids have stronger ionic bonds between their hydrocarbon chains than unsaturated fatty acids do
D) because saturated fatty acids have stronger London dispersion forces between their hydrocarbon chains than unsaturated fatty acids do
A) because saturated fatty acids have stronger repulsive forces between their hydrocarbon chains than unsaturated fatty acids do
B) because saturated fatty acids have stronger hydrogen bonds between their hydrocarbon chains than unsaturated fatty acids do
C) because saturated fatty acids have stronger ionic bonds between their hydrocarbon chains than unsaturated fatty acids do
D) because saturated fatty acids have stronger London dispersion forces between their hydrocarbon chains than unsaturated fatty acids do

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is an unsaturated fatty acid?
A) arachidonic acid
B) palmitic acid
C) stearic acid
D) lauric acid
A) arachidonic acid
B) palmitic acid
C) stearic acid
D) lauric acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following 18 carbon fatty acids will have the lowest melting point?
A) oleic acid
B) linoleic acid
C) linolenic acid
D) stearic acid
A) oleic acid
B) linoleic acid
C) linolenic acid
D) stearic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is true of the sources of fatty acids?
A) Both animals and plants are rich in saturated fatty acids.
B) Both animals and plants are rich in unsaturated fatty acids.
C) Animals are rich in saturated fatty acids, and plants are rich in unsaturated fatty acids.
D) Plants are rich in saturated fatty acids, and animals are rich in unsaturated fatty acids.
A) Both animals and plants are rich in saturated fatty acids.
B) Both animals and plants are rich in unsaturated fatty acids.
C) Animals are rich in saturated fatty acids, and plants are rich in unsaturated fatty acids.
D) Plants are rich in saturated fatty acids, and animals are rich in unsaturated fatty acids.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is true of the most abundant, naturally occurring fatty acids?
A) Nearly all of them contain an even number of carbon atoms.
B) About 70% of them contain an odd number of carbon atoms.
C) Approximately half of them contain an even number of carbon atoms.
D) Nearly all of them contain an odd number of carbon atoms.
A) Nearly all of them contain an even number of carbon atoms.
B) About 70% of them contain an odd number of carbon atoms.
C) Approximately half of them contain an even number of carbon atoms.
D) Nearly all of them contain an odd number of carbon atoms.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following unsaturated acids has the fewest double bonds?
A) arachidonic acid
B) linoleic acid
C) linolenic acid
D) oleic acid
A) arachidonic acid
B) linoleic acid
C) linolenic acid
D) oleic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is true of the most unsaturated fatty acids?
A) The cis isomer predominates the trans isomer.
B) The trans isomer predominates the cis isomer.
C) There is an equal distribution of cis and trans isomers.
D) All are cis isomers.
A) The cis isomer predominates the trans isomer.
B) The trans isomer predominates the cis isomer.
C) There is an equal distribution of cis and trans isomers.
D) All are cis isomers.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following notations corresponds to the fatty acid with the highest melting point?
A) 18:0
B) 18:1
C) 18:2
D) 18:3
A) 18:0
B) 18:1
C) 18:2
D) 18:3

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following fatty acids is unsaturated?
A) arachidonic acid
B) linoleic acid
C) linolenic acid
D) all of these
A) arachidonic acid
B) linoleic acid
C) linolenic acid
D) all of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is the correct statement about stearic acid and linoleic acid?
A) They have same melting point of −5°C.
B) The melting point of stearic acid is lower than that of linoleic acid.
C) The melting point of stearic acid is higher than that of linoleic acid.
D) They have the same melting point of 70°C.
A) They have same melting point of −5°C.
B) The melting point of stearic acid is lower than that of linoleic acid.
C) The melting point of stearic acid is higher than that of linoleic acid.
D) They have the same melting point of 70°C.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following gives the correct order of health risk for the three types of fatty acids?
A) trans > saturated > cis
B) trans > cis > saturated
C) cis > saturated > trans
D) cis > trans > saturated
A) trans > saturated > cis
B) trans > cis > saturated
C) cis > saturated > trans
D) cis > trans > saturated

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
56
Saponification is an example of which type of reaction?
A) esterification
B) ester hydrolysis
C) reduction
D) oxidation
A) esterification
B) ester hydrolysis
C) reduction
D) oxidation

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is true of the melting points of unsaturated fatty acids?
A) The lower the degree of unsaturation, the lower is the melting point.
B) The greater the degree of unsaturation, the lower is the melting point.
C) The greater the degree of unsaturation, the greater is the melting point.
D) The degree of unsaturation has no effect on the melting point.
A) The lower the degree of unsaturation, the lower is the melting point.
B) The greater the degree of unsaturation, the lower is the melting point.
C) The greater the degree of unsaturation, the greater is the melting point.
D) The degree of unsaturation has no effect on the melting point.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following unsaturated fatty acids has the most double bonds?
A) arachidonic acid
B) linoleic acid
C) linolenic acid
D) oleic acid
A) arachidonic acid
B) linoleic acid
C) linolenic acid
D) oleic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
59
How is 3-cyclopentene-carboxylic acid converted to 4-hydroxymethyl-cyclopentene?
A) by heating to a high temperature to undergo decarboxylation
B) by treating with sodium bicarbonate
C) by reducing with LiAlH4 followed by hydrolysis
D) by acid-catalyzed reaction with ethanol
A) by heating to a high temperature to undergo decarboxylation
B) by treating with sodium bicarbonate
C) by reducing with LiAlH4 followed by hydrolysis
D) by acid-catalyzed reaction with ethanol

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is a saturated fatty acid?
A) arachidonic acid
B) oleic acid
C) palmitic acid
D) linoleic acid
A) arachidonic acid
B) oleic acid
C) palmitic acid
D) linoleic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
61
In which form will carboxylic acid, RCOOH, be present in an aqueous solution with pH of the solution equal to the pKa of the acid?
A) primarily as RCOOH(aq)
B) primarily as RCOO− (aq)
C) equal amounts of RCOOH(aq) and RCOO− (aq)
D) neither RCOOH(aq) nor RCOO− (aq)
A) primarily as RCOOH(aq)
B) primarily as RCOO− (aq)
C) equal amounts of RCOOH(aq) and RCOO− (aq)
D) neither RCOOH(aq) nor RCOO− (aq)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
62
In a soap micelle, which of the following is true?
A) The hydrophilic region is on the surface and the hydrophobic region is in the interior.
B) The hydrophobic region is on the surface and the hydrophilic region is in the interior.
C) The hydrophilic and hydrophobic regions are uniformly distributed throughout the surface of the micelle.
D) When the micelle is formed, the hydrophilic and hydrophobic regions interact and neutralize one another.
A) The hydrophilic region is on the surface and the hydrophobic region is in the interior.
B) The hydrophobic region is on the surface and the hydrophilic region is in the interior.
C) The hydrophilic and hydrophobic regions are uniformly distributed throughout the surface of the micelle.
D) When the micelle is formed, the hydrophilic and hydrophobic regions interact and neutralize one another.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is a detergent molecule?
A) sodium alkylbenzenesulfonate
B) sodium silicate
C) sodium perborate tetrahydrate
D) none of these
A) sodium alkylbenzenesulfonate
B) sodium silicate
C) sodium perborate tetrahydrate
D) none of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the cations that produce water-insoluble salts when mixed with water.
A) Ca2+ ions
B) K+ ions
C) both Ca2+ ions and K+ ions
D) neither Ca2+ ions nor K+ ions
A) Ca2+ ions
B) K+ ions
C) both Ca2+ ions and K+ ions
D) neither Ca2+ ions nor K+ ions

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following correctly describes the reagent(s) used to manufacture a detergent from a linear alkylbenzene precursor?
A) NaOH only
B) H2SO4 only
C) NaOH followed by H2SO4
D) H2SO4 followed by NaOH
A) NaOH only
B) H2SO4 only
C) NaOH followed by H2SO4
D) H2SO4 followed by NaOH

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following compounds is the strongest acid?
A) acetic acid
B) chloroacetic acid
C) dichloroacetic acid
D) trichloroacetic acid
A) acetic acid
B) chloroacetic acid
C) dichloroacetic acid
D) trichloroacetic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
67
What is formed by the reaction of benzoic acid with sodium hydroxide?
A) sodium perborate tetrahydrate
B) sodium hydroxybenzoate
C) sodium benzenesulfonate
D) sodium benzoate
A) sodium perborate tetrahydrate
B) sodium hydroxybenzoate
C) sodium benzenesulfonate
D) sodium benzoate

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
68
Identify the Henderson-Hasselbalch equation.
A)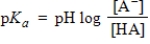
B)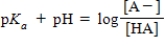
C)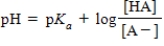
D)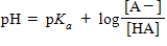
A)
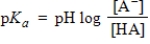
B)
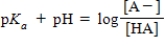
C)
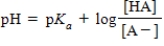
D)
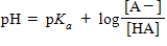

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
69
Given that the electron-withdrawing ability of a substituent falls off with the distance, rank order the following carboxylic acids from the strongest acid to the weakest acid. 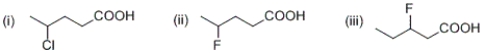
A) (i) > (ii) > (iii)
B) (iii) > (ii) > (i)
C) (ii) > (iii) > (i)
D) (ii) > (i) > (iii)

A) (i) > (ii) > (iii)
B) (iii) > (ii) > (i)
C) (ii) > (iii) > (i)
D) (ii) > (i) > (iii)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following cations are present in hard water?
A) Ca2+ ions
B) Fe3+ ions
C) Mg2+ ions
D) all of these
A) Ca2+ ions
B) Fe3+ ions
C) Mg2+ ions
D) all of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following reagents is required in saponification?
A) an oxidizing agent
B) a strong base
C) a reducing agent
D) a weak acid
A) an oxidizing agent
B) a strong base
C) a reducing agent
D) a weak acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
72
The reactions of a carboxylic acid with which of the following reagents produces a gas?
A) NaHCO3
B) NaOH
C) both NaHCO3 and NaOH
D) neither NaHCO3 nor NaOH
A) NaHCO3
B) NaOH
C) both NaHCO3 and NaOH
D) neither NaHCO3 nor NaOH

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following can be used as a fungal growth inhibitor?
A) calcium propanoate
B) sodium benzoate
C) both calcium propanoate and sodium benzoate
D) neither calcium propanoate nor sodium benzoate
A) calcium propanoate
B) sodium benzoate
C) both calcium propanoate and sodium benzoate
D) neither calcium propanoate nor sodium benzoate

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
74
What happens when oil is added to a soap solution?
A) Oil adsorbs on the outer surface of the soap micelles.
B) Oil dissolves in the nonpolar hydrocarbon inner parts of the soap micelles.
C) Both of these are correct.
D) Neither of these is correct.
A) Oil adsorbs on the outer surface of the soap micelles.
B) Oil dissolves in the nonpolar hydrocarbon inner parts of the soap micelles.
C) Both of these are correct.
D) Neither of these is correct.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
75
In which form will carboxylic acid, RCOOH, be present in an aqueous solution with pH greater than 7.0?
A) primarily as RCOOH(aq)
B) primarily as RCOO− (aq)
C) equal amounts of RCOOH(aq) and RCOO− (aq)
D) neither RCOOH(aq) nor RCOO− (aq)
A) primarily as RCOOH(aq)
B) primarily as RCOO− (aq)
C) equal amounts of RCOOH(aq) and RCOO− (aq)
D) neither RCOOH(aq) nor RCOO− (aq)

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
76
In the Henderson-Hasselbalch equation, when will the pH be equal to the pKa?
A) when [A−] = 0.5 × [HA]
B) when [A−] = 1 × [HA]
C) when [HA]= 3 × [A−]
D) when [A−] = 2 × [HA]
A) when [A−] = 0.5 × [HA]
B) when [A−] = 1 × [HA]
C) when [HA]= 3 × [A−]
D) when [A−] = 2 × [HA]

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
77
What is the difference between stearic acid and linoleic acid?
A) Stearic acid has 18 carbon atoms, whereas linoleic acid has 16 carbon atoms.
B) Stearic acid is unsaturated, whereas linoleic acid is saturated.
C) Stearic acid has 18 carbon atoms, whereas linoleic acid has 20 carbon atoms.
D) Stearic acid is saturated, whereas linoleic acid is unsaturated.
A) Stearic acid has 18 carbon atoms, whereas linoleic acid has 16 carbon atoms.
B) Stearic acid is unsaturated, whereas linoleic acid is saturated.
C) Stearic acid has 18 carbon atoms, whereas linoleic acid has 20 carbon atoms.
D) Stearic acid is saturated, whereas linoleic acid is unsaturated.

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is used as a bleaching agent in detergent preparations?
A) sodium alkylbenzenesulfonate
B) sodium silicate
C) sodium perborate tetrahydrate
D) none of these
A) sodium alkylbenzenesulfonate
B) sodium silicate
C) sodium perborate tetrahydrate
D) none of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is a byproduct in the production of soaps?
A) glycerol
B) triglycerides
C) glyceric acid
D) none of these
A) glycerol
B) triglycerides
C) glyceric acid
D) none of these

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following is used by dentists to cauterize gums?
A) acetic acid
B) chloroacetic acid
C) dichloroacetic acid
D) trichloroacetic acid
A) acetic acid
B) chloroacetic acid
C) dichloroacetic acid
D) trichloroacetic acid

Unlock Deck
Unlock for access to all 115 flashcards in this deck.
Unlock Deck
k this deck


