Deck 16: Aldehydes and Ketones
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/102
Play
Full screen (f)
Deck 16: Aldehydes and Ketones
1
Which of the following is true of the IUPAC nomenclature of ketones?
A) The parent name is based on the longest carbon chain that contains the carbonyl carbon.
B) The oxygen atom is always bonded to the first carbon.
C) The parent name is based on the longest carbon chain that contains the carbonyl carbon, and the oxygen atom is always bonded to the first carbon.
D) None of these
A) The parent name is based on the longest carbon chain that contains the carbonyl carbon.
B) The oxygen atom is always bonded to the first carbon.
C) The parent name is based on the longest carbon chain that contains the carbonyl carbon, and the oxygen atom is always bonded to the first carbon.
D) None of these
A
2
What is the IUPAC name of the following compound? 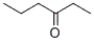
A) 4-hexanone
B) 3-hexanone
C) ethyl propyl ketone
D) propyl ethyl ketone

A) 4-hexanone
B) 3-hexanone
C) ethyl propyl ketone
D) propyl ethyl ketone
B
3
What is the smallest number of carbon atoms that can be present in an aldehyde?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4
A
4
If pairs of enantiomers are only counted once, how many different aldehydes have the molecular formula C5H10O?
A) 2
B) 3
C) 4
D) 5
A) 2
B) 3
C) 4
D) 5

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
5
What is the IUPAC name of the following compound? 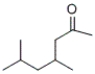
A) 2,4-dimethyl-6-heptanone
B) 4,6-dimethyl-2-heptanone
C) 4,6-dimethylheptanone
D) 2,4-dimethylhexanal

A) 2,4-dimethyl-6-heptanone
B) 4,6-dimethyl-2-heptanone
C) 4,6-dimethylheptanone
D) 2,4-dimethylhexanal

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
6
What is the IUPAC name of the following compound? 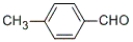
A) 4-aldyltoluene
B) 4-toluylaldehyde
C) 1-methylbenzaldehyde
D) 4-methylbenzaldehyde

A) 4-aldyltoluene
B) 4-toluylaldehyde
C) 1-methylbenzaldehyde
D) 4-methylbenzaldehyde

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
7
What is the IUPAC name of the following compound? 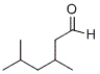
A) 2,4-dimethyl-6-hexanal
B) 2-aldehydomethyl-4-methylpentane
C) 3,5-dimethyl-2-hexanal
D) 3,5-dimethylhexanal

A) 2,4-dimethyl-6-hexanal
B) 2-aldehydomethyl-4-methylpentane
C) 3,5-dimethyl-2-hexanal
D) 3,5-dimethylhexanal

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
8
What is the IUPAC name of the following compound? 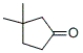
A) 1,1-dimethyl-3-pentanone
B) 3,3-methyl-1-pentanone
C) 3,3-dimethylcyclopentanone
D) 1,1-dimethyl-3-cyclopentanone

A) 1,1-dimethyl-3-pentanone
B) 3,3-methyl-1-pentanone
C) 3,3-dimethylcyclopentanone
D) 1,1-dimethyl-3-cyclopentanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
9
Which structural feature is common to aldehydes and ketones?
A) an oxygen atom bonded to both a carbon atom and a hydrogen atom
B) an oxygen atom bonded to two carbon atoms
C) an oxygen atom double bonded to a carbon atom
D) two oxygen atoms bonded to the same carbon atom
A) an oxygen atom bonded to both a carbon atom and a hydrogen atom
B) an oxygen atom bonded to two carbon atoms
C) an oxygen atom double bonded to a carbon atom
D) two oxygen atoms bonded to the same carbon atom

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
10
In the IUPAC name 2-methyl-2-propenal, what do the numbers represent?
A) the position of the methyl substituent and the position of the carbonyl group
B) the position of the methyl substituent and the position of the double bond
C) the position of the carbonyl group and the position of the double bond
D) the position of the carbonyl group and the position of the triple bond
A) the position of the methyl substituent and the position of the carbonyl group
B) the position of the methyl substituent and the position of the double bond
C) the position of the carbonyl group and the position of the double bond
D) the position of the carbonyl group and the position of the triple bond

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is true of the IUPAC nomenclature of aldehydes?
A) The parent name is based on the longest carbon chain that contains the carbonyl carbon.
B) The oxygen atom is always bonded to the first carbon.
C) The parent name is based on the longest carbon chain that contains the carbonyl carbon and the oxygen atom is always bonded to the first carbon.
D) None of these
A) The parent name is based on the longest carbon chain that contains the carbonyl carbon.
B) The oxygen atom is always bonded to the first carbon.
C) The parent name is based on the longest carbon chain that contains the carbonyl carbon and the oxygen atom is always bonded to the first carbon.
D) None of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a correct IUPAC name?
A) 1-methylpropanal
B) 2-methylpropanal
C) 3-methylpropanal
D) none of these
A) 1-methylpropanal
B) 2-methylpropanal
C) 3-methylpropanal
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
13
Identify the IUPAC name of the given compound. 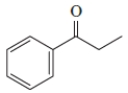
A) 1-propylbenzophenone
B) 1-phenyl-1-propanone
C) 1-propylphenylketone
D) 1-phenylpropyl ketone

A) 1-propylbenzophenone
B) 1-phenyl-1-propanone
C) 1-propylphenylketone
D) 1-phenylpropyl ketone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
14
What is the IUPAC name of the following compound? 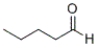
A) 1-pentaldehyde
B) pentanal
C) pentanoate
D) pentanone

A) 1-pentaldehyde
B) pentanal
C) pentanoate
D) pentanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is a correct IUPAC name?
A) 1-methylpropanal
B) 2-ethylpropanal
C) 2,2-dimethylpropanal
D) none of these
A) 1-methylpropanal
B) 2-ethylpropanal
C) 2,2-dimethylpropanal
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
16
What is the smallest number of carbon atoms that can be present in a ketone?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
17
Which functional group is found in ketones?
A) R-CHO
B) R-CH2OH
C) R-COOH
D) R-COR'
A) R-CHO
B) R-CH2OH
C) R-COOH
D) R-COR'

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
18
How many ketone functional groups are present in the following compound? 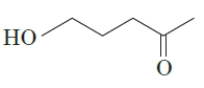
A) 2
B) 1
C) 4
D) 5

A) 2
B) 1
C) 4
D) 5

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
19
How many different aldehydes have the molecular formula C4H8O?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
20
Which functional group is found in aldehydes?
A) R-CHO
B) R-CH2OH
C) R-COOH
D) R-COR'
A) R-CHO
B) R-CH2OH
C) R-COOH
D) R-COR'

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
21
What is the IUPAC name of propyl butyl ketone?
A) 4-octanone
B) 2-methyl-4-heptanone
C) 3-methyl-4-heptanone
D) 2,2-dimethyl-3-hexanone
A) 4-octanone
B) 2-methyl-4-heptanone
C) 3-methyl-4-heptanone
D) 2,2-dimethyl-3-hexanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
22
What is the IUPAC name of propyl isobutyl ketone?
A) 4-octanone
B) 2-methyl-4-heptanone
C) 3-methyl-4-heptanone
D) 2,2-dimethyl-3-hexanone
A) 4-octanone
B) 2-methyl-4-heptanone
C) 3-methyl-4-heptanone
D) 2,2-dimethyl-3-hexanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
23
How many different ketones have the molecular formula C5H10O?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following compounds has the lowest boiling point?
A) butane
B) propanal
C) ethyl methyl ether
D) 1-propanol
A) butane
B) propanal
C) ethyl methyl ether
D) 1-propanol

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds has the highest boiling point?
A) butane
B) propanal
C) ethyl methyl ether
D) 1-propanol
A) butane
B) propanal
C) ethyl methyl ether
D) 1-propanol

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following has intermolecular hydrogen bonds?
A) pure formaldehyde
B) pure acetone
C) a solution of formaldehyde in water
D) none of these
A) pure formaldehyde
B) pure acetone
C) a solution of formaldehyde in water
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
27
What is the IUPAC name of the following compound? 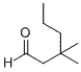
A) 3-methyl-3-propylbutanal
B) 3-methyl-3-propyl-1-butanal
C) 3,3-dimethylhexanal
D) 3,3-methyl-1-hexanal

A) 3-methyl-3-propylbutanal
B) 3-methyl-3-propyl-1-butanal
C) 3,3-dimethylhexanal
D) 3,3-methyl-1-hexanal

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
28
What is the common name of 2-octanone?
A) methyl heptyl ketone
B) heptyl methyl ketone
C) methyl hexyl ketone
D) hexyl methyl ketone
A) methyl heptyl ketone
B) heptyl methyl ketone
C) methyl hexyl ketone
D) hexyl methyl ketone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
29
What is the IUPAC name of propyl tert-butyl ketone?
A) 4-octanone
B) 2-methyl-4-heptanone
C) 3-methyl-4-heptanone
D) 2,2-dimethyl-3-hexanone
A) 4-octanone
B) 2-methyl-4-heptanone
C) 3-methyl-4-heptanone
D) 2,2-dimethyl-3-hexanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
30
What is the correct order of boiling points of the following compounds? 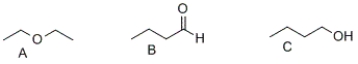
A) A > B > C
B) C > B > A
C) B > A > C
D) A > C > B

A) A > B > C
B) C > B > A
C) B > A > C
D) A > C > B

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
31
What is the IUPAC name of HOCH2CH2CHO?
A) 1-hydroxy-3-propanal
B) 2-hydroxypropanal
C) 3-hydroxypropanal
D) 1,3-propanedial
A) 1-hydroxy-3-propanal
B) 2-hydroxypropanal
C) 3-hydroxypropanal
D) 1,3-propanedial

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
32
What is the correct order of boiling points of the following compounds? 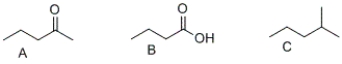
A) A > B > C
B) C > B > A
C) B > A > C
D) A > C > B

A) A > B > C
B) C > B > A
C) B > A > C
D) A > C > B

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
33
What is the IUPAC name of the following compound? 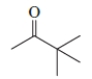
A) 1,1,1-trimethyl-2-propanone
B) 3,3-dimethyl-2-butanone
C) 3,3,3-trimethyl-2-propanone
D) 2,2-dimethyl-3-butanone

A) 1,1,1-trimethyl-2-propanone
B) 3,3-dimethyl-2-butanone
C) 3,3,3-trimethyl-2-propanone
D) 2,2-dimethyl-3-butanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
34
What is the IUPAC name of propyl sec-butyl ketone?
A) 4-octanone
B) 2-methyl-4-heptanone
C) 3-methyl-4-heptanone
D) 2,2-dimethyl-3-hexanone
A) 4-octanone
B) 2-methyl-4-heptanone
C) 3-methyl-4-heptanone
D) 2,2-dimethyl-3-hexanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
35
What is the IUPAC name of the following compound? 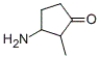
A) 2-methyl-3-amino-1-cyclopentanone
B) 3-amine-2-methyl-1-cyclopentanone
C) 2-methyl-3-aminocyclopentanone
D) 3-amino-2-methylcyclopentanone

A) 2-methyl-3-amino-1-cyclopentanone
B) 3-amine-2-methyl-1-cyclopentanone
C) 2-methyl-3-aminocyclopentanone
D) 3-amino-2-methylcyclopentanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following has intermolecular hydrogen bonds?
A) pure acetone
B) pure formaldehyde
C) a mixture of acetone and formaldehyde
D) none of these
A) pure acetone
B) pure formaldehyde
C) a mixture of acetone and formaldehyde
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following has intermolecular hydrogen bonds?
A) a solution of acetone in water
B) a solution of formaldehyde in water
C) a solution of acetone and formaldehyde in water
D) all of these
A) a solution of acetone in water
B) a solution of formaldehyde in water
C) a solution of acetone and formaldehyde in water
D) all of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
38
What is the common name of 3-methyl-4-heptanone?
A) propyl butyl ketone
B) propyl sec-butyl ketone
C) propyl tert-butyl ketone
D) propyl isobutyl ketone
A) propyl butyl ketone
B) propyl sec-butyl ketone
C) propyl tert-butyl ketone
D) propyl isobutyl ketone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
39
What is the IUPAC name of the following compound? 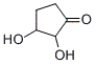
A) 2,3-dihydroxycyclopentanone
B) 1,2-dihydroxy-3-cyclopentanone
C) cyclopentanone-1,2-diol
D) cyclopentanone-2,3-diol

A) 2,3-dihydroxycyclopentanone
B) 1,2-dihydroxy-3-cyclopentanone
C) cyclopentanone-1,2-diol
D) cyclopentanone-2,3-diol

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the IUPAC name of the given compound. 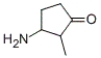
A) 2-methyl-3-amino-1-cyclopentanone
B) 1-amino-2-methyl-3-cyclopentanone
C) 3-amino-2-methyl-1-cyclopentanone
D) 2-amino-1-methyl-2-cyclopentanone

A) 2-methyl-3-amino-1-cyclopentanone
B) 1-amino-2-methyl-3-cyclopentanone
C) 3-amino-2-methyl-1-cyclopentanone
D) 2-amino-1-methyl-2-cyclopentanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following compounds is the most soluble in water?
A) butanal
B) heptanal
C) hexanal
D) pentanal
A) butanal
B) heptanal
C) hexanal
D) pentanal

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following statements is true of aldehydes and ketones?
A) Both aldehydes and ketones are easily oxidized.
B) Neither aldehydes nor ketones are easily oxidized.
C) Aldehydes are easily oxidized, but ketones cannot be readily oxidized.
D) Ketones are easily oxidized, but aldehydes cannot be readily oxidized.
A) Both aldehydes and ketones are easily oxidized.
B) Neither aldehydes nor ketones are easily oxidized.
C) Aldehydes are easily oxidized, but ketones cannot be readily oxidized.
D) Ketones are easily oxidized, but aldehydes cannot be readily oxidized.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following will give a positive test with Tollens' reagent?
A) aldehydes
B) ketones
C) aldehydes and ketones
D) none of these
A) aldehydes
B) ketones
C) aldehydes and ketones
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following compounds is the most soluble in water?
A) 2-butanone
B) 1-hydroxy-2-butanone
C) butanal
D) 2,3-dihydroxybutanal
A) 2-butanone
B) 1-hydroxy-2-butanone
C) butanal
D) 2,3-dihydroxybutanal

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is true of liquid aldehydes?
A) They are not readily oxidized.
B) They are not oxidized by O2 in the air but are oxidized by stronger oxidizing agents.
C) They are readily oxidized by O2 in the air.
D) Their most important oxidation reaction is combustion.
A) They are not readily oxidized.
B) They are not oxidized by O2 in the air but are oxidized by stronger oxidizing agents.
C) They are readily oxidized by O2 in the air.
D) Their most important oxidation reaction is combustion.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
46
Which product will be formed by the treatment of the following compound with silver nitrate and ammonia in water, followed by acidification with HCl? 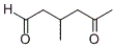
A)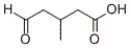
B)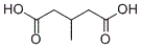
C)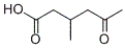
D) none of these

A)

B)

C)

D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is an oxidizing agent?
A) NADH
B) H2
C) O2
D) none of these
A) NADH
B) H2
C) O2
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following compounds has the highest water solubility?
A) butanal
B) 1-butanol
C) 1-hexanol
D) hexanal
A) butanal
B) 1-butanol
C) 1-hexanol
D) hexanal

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following will generally give a positive test with Tollens' reagent?
A) carboxylic acids
B) ketones
C) both carboxylic acids and ketones
D) none of these
A) carboxylic acids
B) ketones
C) both carboxylic acids and ketones
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is true of ketones?
A) Most ketones are odorless.
B) Most ketones have mild, fishy odors.
C) Most ketones have strong, pleasant odors.
D) Most ketones have strong, unpleasant odors.
A) Most ketones are odorless.
B) Most ketones have mild, fishy odors.
C) Most ketones have strong, pleasant odors.
D) Most ketones have strong, unpleasant odors.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following compounds has the lowest boiling point?
A) acetone
B) formaldehyde
C) 3-octanone
D) cyclopentanone
A) acetone
B) formaldehyde
C) 3-octanone
D) cyclopentanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following compounds is obtained by the oxidation of hexanal?
A) hexanol
B) 2-hexanone
C) hexanoic acid
D) none of these
A) hexanol
B) 2-hexanone
C) hexanoic acid
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following compounds has the lowest water solubility?
A) butanal
B) heptanal
C) hexanal
D) pentanal
A) butanal
B) heptanal
C) hexanal
D) pentanal

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following compounds has the highest boiling point?
A) 2-butanone
B) 2-heptanone
C) 2-hexanone
D) 2-pentanone
A) 2-butanone
B) 2-heptanone
C) 2-hexanone
D) 2-pentanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following will generally give a positive test with Tollens' reagent?
A) aldehydes
B) carboxylic acids
C) both aldehydes and carboxylic acids
D) none of these
A) aldehydes
B) carboxylic acids
C) both aldehydes and carboxylic acids
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
56
What is the final product obtained when 2-hexanone is treated with potassium dichromate in the presence of sulfuric acid?
A) an equilibrium mixture of 2-hexanone and hemiacetal
B) hexanol
C) hexanoic acid
D) none of these, as 2-hexanone does not undergo oxidation readily
A) an equilibrium mixture of 2-hexanone and hemiacetal
B) hexanol
C) hexanoic acid
D) none of these, as 2-hexanone does not undergo oxidation readily

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following compounds has the lowest boiling point?
A) 2-butanone
B) 2-heptanone
C) 2-hexanone
D) 2-pentanone
A) 2-butanone
B) 2-heptanone
C) 2-hexanone
D) 2-pentanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following compounds has the highest boiling point?
A) acetone
B) butane
C) ethyl methyl ether
D) 2-propanol
A) acetone
B) butane
C) ethyl methyl ether
D) 2-propanol

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is the least soluble in water?
A) 2-butanone
B) 1-hydroxy-2-butanone
C) 2,3-dihydroxybutanal
D) 2-pentanone
A) 2-butanone
B) 1-hydroxy-2-butanone
C) 2,3-dihydroxybutanal
D) 2-pentanone

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following compounds will undergo oxidation using potassium dichromate to form a carboxylic acid? 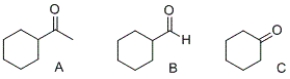
A) A and B only
B) A and C only
C) B only
D) C only

A) A and B only
B) A and C only
C) B only
D) C only

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following pairs of reactants produces a hemiacetal?
A) aldehyde and alcohol
B) aldehyde and amine
C) aldehyde and carboxylic acid
D) aldehyde and water
A) aldehyde and alcohol
B) aldehyde and amine
C) aldehyde and carboxylic acid
D) aldehyde and water

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is obtained from the reaction of 2-butanone with H2 in the presence of a transition metal catalyst?
A) 1-butene
B) 2-butene
C) 1-butanol
D) 2-butanol
A) 1-butene
B) 2-butene
C) 1-butanol
D) 2-butanol

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following compounds contain an acetal functional group? 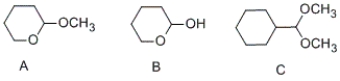
A) A and B
B) A and C
C) B and C
D) only C

A) A and B
B) A and C
C) B and C
D) only C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
64
What is produced by the reduction of pyruvate in the body under anaerobic conditions?
A) acetate
B) acetyl CoA
C) lactate
D) pyruvate
A) acetate
B) acetyl CoA
C) lactate
D) pyruvate

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
65
Of the various -OH groups in the following carbohydrate, how many are part of the hemiacetal functional group? 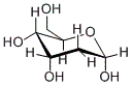
A) 4
B) 1
C) 2
D) 3

A) 4
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
66
In biological systems, which of the following compounds reduces aldehydes and ketones?
A) NADH2
B) NADH
C) NAD+
D) none of these
A) NADH2
B) NADH
C) NAD+
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
67
Which types of biologically important molecules include many cyclic hemiacetals?
A) carbohydrates
B) lipids
C) proteins
D) none of these
A) carbohydrates
B) lipids
C) proteins
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following pairs of reactants produces a hemiacetal?
A) ketone and alcohol
B) ketone and aldehyde
C) ketone and carboxylic acid
D) none of these
A) ketone and alcohol
B) ketone and aldehyde
C) ketone and carboxylic acid
D) none of these

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following compounds is obtained from the reaction of pentanal with H2 in the presence of a transition metal catalyst?
A) pentene
B) 1-pentanol
C) 2-pentanol
D) pentanoic acid
A) pentene
B) 1-pentanol
C) 2-pentanol
D) pentanoic acid

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
70
What is the minimum number of oxygen atoms present in a hemiacetal?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
71
What is the minimum number of oxygen atoms present in an acetal?
A) 0
B) 1
C) 2
D) 3
A) 0
B) 1
C) 2
D) 3

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following statements is correct regarding the reduction of an unsaturated aldehyde or ketone using H2 in the presence of a transition metal catalyst?
A) Both the carbon-carbon and the carbon-oxygen double bonds are reduced at the same rate.
B) The carbon-carbon double bond is reduced more rapidly than the carbon-oxygen double bond.
C) The carbon-oxygen double bond is reduced more rapidly than the carbon-carbon double bond.
D) Unsaturated aldehydes and ketones do not undergo any reduction reactions under these conditions.
A) Both the carbon-carbon and the carbon-oxygen double bonds are reduced at the same rate.
B) The carbon-carbon double bond is reduced more rapidly than the carbon-oxygen double bond.
C) The carbon-oxygen double bond is reduced more rapidly than the carbon-carbon double bond.
D) Unsaturated aldehydes and ketones do not undergo any reduction reactions under these conditions.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is true of hemiacetals?
A) The most stable hemiacetals are noncyclic.
B) Hemiacetals react with alcohols to form acetates.
C) Hemiacetals react with alcohols to form acetals.
D) Hemiacetals contain two carbonyl groups in their stable form.
A) The most stable hemiacetals are noncyclic.
B) Hemiacetals react with alcohols to form acetates.
C) Hemiacetals react with alcohols to form acetals.
D) Hemiacetals contain two carbonyl groups in their stable form.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following compounds is obtained from the reduction of butanal using NaBH4 followed by treatment with water?
A) butene
B) 1-butanol
C) 2-butanol
D) butanoic acid
A) butene
B) 1-butanol
C) 2-butanol
D) butanoic acid

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following compounds contain a hemiacetal functional group? 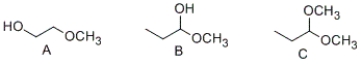
A) Α and B
B) Β and C
C) only B
D) only C

A) Α and B
B) Β and C
C) only B
D) only C

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following statements is true of hemiacetals?
A) All hemiacetals are generally stable.
B) All hemiacetals are unstable because of the presence of two carbonyl groups.
C) The functional group of hemiacetals is a carbon bonded to one -OH group and one -OR group.
D) Hemiacetals have two -OH groups bonded to the same carbon.
A) All hemiacetals are generally stable.
B) All hemiacetals are unstable because of the presence of two carbonyl groups.
C) The functional group of hemiacetals is a carbon bonded to one -OH group and one -OR group.
D) Hemiacetals have two -OH groups bonded to the same carbon.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is the first step in the acid catalyzed formation of an acetal?
A) A proton is added to the carbonyl oxygen, giving a resonance-stabilized cation.
B) An electrophile and a nucleophile react to form a new covalent bond.
C) A proton is transferred from one oxygen to another oxygen.
D) A tertiary carbocation is formed by breaking the bond between a hydroxyl group and a carbon.
A) A proton is added to the carbonyl oxygen, giving a resonance-stabilized cation.
B) An electrophile and a nucleophile react to form a new covalent bond.
C) A proton is transferred from one oxygen to another oxygen.
D) A tertiary carbocation is formed by breaking the bond between a hydroxyl group and a carbon.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following is obtained from the reaction of 2-pentanone with NaBH4 followed by the addition of water?
A) pentane
B) 1-pentanol
C) 2-pentanol
D) pentanoic acid
A) pentane
B) 1-pentanol
C) 2-pentanol
D) pentanoic acid

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
79
Which of following statements is correct regarding the following chemical reaction? 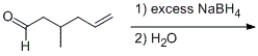
A) Both the carbon-carbon and the carbon-oxygen double bonds will be reduced.
B) Only the carbon-carbon double bond will be reduced.
C) Only the carbon-oxygen double bond will be reduced.
D) Neither the carbon-carbon double bond nor the carbon-oxygen double bond will be reduced.
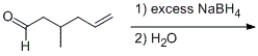
A) Both the carbon-carbon and the carbon-oxygen double bonds will be reduced.
B) Only the carbon-carbon double bond will be reduced.
C) Only the carbon-oxygen double bond will be reduced.
D) Neither the carbon-carbon double bond nor the carbon-oxygen double bond will be reduced.

Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following compounds produces 1-pentanol upon reduction with an H2/metal catalyst?
A)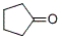
B)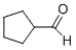
C)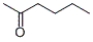
D)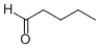
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 102 flashcards in this deck.
Unlock Deck
k this deck


