Deck 11: Alkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/142
Play
Full screen (f)
Deck 11: Alkanes
1
Which type of hydrocarbons are alkanes?
A) heterocyclic
B) aromatic
C) saturated
D) unsaturated
A) heterocyclic
B) aromatic
C) saturated
D) unsaturated
C
2
Which of the following is the molecular formula of heptane?
A) C5H12
B) C6H14
C) C7H16
D) C8H18
A) C5H12
B) C6H14
C) C7H16
D) C8H18
C
3
Octane is an alkane having eight carbon atoms in its carbon chain. What is the total number of hydrogen atoms in octane?
A) 22
B) 16
C) 18
D) 20
A) 22
B) 16
C) 18
D) 20
C
4
Which of the following compounds contains a carbon-carbon double bond?
A) ethane
B) ethylene
C) methylene
D) acetylene
A) ethane
B) ethylene
C) methylene
D) acetylene

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
5
What is the name given to a -CH2- group?
A) methane
B) methoxy
C) methylene
D) methyl
A) methane
B) methoxy
C) methylene
D) methyl

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds is an alkane?
A) C5H10
B) C6H10
C) C7H18
D) C8H18
A) C5H10
B) C6H10
C) C7H18
D) C8H18

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
7
Unbranched hydrocarbons are also called straight-chain hydrocarbons. Which of the following is true of unbranched hydrocarbons?
A) The C-C-C bond angle is 180° in unbranched hydrocarbons.
B) Each carbon atom is connected to not more than two other carbon atoms.
C) The C-C-C bond angle is 180o in unbranched hydrocarbons and each carbon atom is connected to not more than two other carbon atoms.
D) None of these.
A) The C-C-C bond angle is 180° in unbranched hydrocarbons.
B) Each carbon atom is connected to not more than two other carbon atoms.
C) The C-C-C bond angle is 180o in unbranched hydrocarbons and each carbon atom is connected to not more than two other carbon atoms.
D) None of these.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the molecular formula of nonane?
A) C9H22
B) C9H20
C) C9H21
D) C9H18
A) C9H22
B) C9H20
C) C9H21
D) C9H18

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following represents the molecular formula of an alkane?
A) C10H32
B) C10H40
C) C10H42
D) C10H22
A) C10H32
B) C10H40
C) C10H42
D) C10H22

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following compounds contains only carbon-carbon single bonds?
A) ethane
B) ethylene
C) benzene
D) acetylene
A) ethane
B) ethylene
C) benzene
D) acetylene

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds contain one or more benzene-like ring structures?
A) alkanes
B) alkenes
C) alkynes
D) arenes
A) alkanes
B) alkenes
C) alkynes
D) arenes

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following hydrocarbons contains the maximum number of carbon atoms?
A) butane
B) pentane
C) ethane
D) propane
A) butane
B) pentane
C) ethane
D) propane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following represents the molecular formula of a noncyclic alkane?
A) C5H10
B) C6H12
C) C4H8
D) none of these
A) C5H10
B) C6H12
C) C4H8
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
14
Decane is an alkane having ten carbon atoms in its carbon chain. What is the total number of hydrogen atoms in decane?
A) 12
B) 24
C) 22
D) 18
A) 12
B) 24
C) 22
D) 18

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is the molecular formula of hexane?
A) C6H6
B) C6H10
C) C6H14
D) C6H16
A) C6H6
B) C6H10
C) C6H14
D) C6H16

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
16
Why are alkanes called saturated hydrocarbons?
A) because alkanes are used as solvents for most organic compounds
B) because alkanes are highly reactive organic compounds
C) because alkanes contain only carbon-carbon single bonds
D) because alkanes are found in animal fats and plant oils
A) because alkanes are used as solvents for most organic compounds
B) because alkanes are highly reactive organic compounds
C) because alkanes contain only carbon-carbon single bonds
D) because alkanes are found in animal fats and plant oils

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds contains a carbon-carbon triple bond?
A) ethane
B) ethylene
C) methylene
D) acetylene
A) ethane
B) ethylene
C) methylene
D) acetylene

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is the molecular formula of heptane?
A) C7H12
B) C7H16
C) C7H22
D) C7H14
A) C7H12
B) C7H16
C) C7H22
D) C7H14

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
19
What is the H-C-H bond angle in a methane molecule?
A) 104°
B) 120°
C) 105.9°
D) 109.5°
A) 104°
B) 120°
C) 105.9°
D) 109.5°

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following correctly describes the relationship between butane and 2-methylpropane?
A) They have the same molecular formula.
B) They have the same chemical properties.
C) They have the same physical properties.
D) All of these are true.
A) They have the same molecular formula.
B) They have the same chemical properties.
C) They have the same physical properties.
D) All of these are true.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following compounds are representations of the same molecule? (i) CH3CH2CH2CH2CH2CH3
(ii)
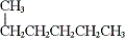 (iii)
(iii)
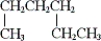
A) only (i) and (ii)
B) only (i) and (iii)
C) only (ii) and (iii)
D) all of these
(ii)
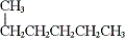 (iii)
(iii) 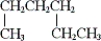
A) only (i) and (ii)
B) only (i) and (iii)
C) only (ii) and (iii)
D) all of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
22
How many different substituent groups can be obtained by removing a hydrogen atom from butane?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
23
Hexane and 2-methylpentane are constitutional isomers. Which of the following statements is true of these isomers?
A) Hexane and 2-methylpentane have the same melting point.
B) Hexane and 2-methylpentane have the same boiling point.
C) Hexane and 2-methylpentane have the same molecular formula.
D) Hexane and 2-methylpentane have the same molecular structure.
A) Hexane and 2-methylpentane have the same melting point.
B) Hexane and 2-methylpentane have the same boiling point.
C) Hexane and 2-methylpentane have the same molecular formula.
D) Hexane and 2-methylpentane have the same molecular structure.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is a constitutional isomer of hexane?
A) CH3CH2CH2CH2CH2CH2CH2CH3
B)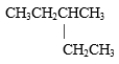
C) both CH3CH2CH2CH2CH2CH2CH2CH3 and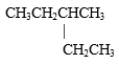
D) none of these
A) CH3CH2CH2CH2CH2CH2CH2CH3
B)
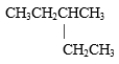
C) both CH3CH2CH2CH2CH2CH2CH2CH3 and
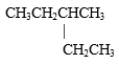
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
25
How many constitutional isomers have the molecular formula C6H14?
A) 4
B) 5
C) 6
D) 7
A) 4
B) 5
C) 6
D) 7

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is true of a hydrocarbon with a branched chain?
A) The longest chain of carbon atoms is the parent chain, and its name becomes the root name.
B) The shortest chain of carbon atoms is the parent chain, and its name becomes the root name.
C) The longest chain of carbon atoms is the parent chain, and its name becomes the root name and the shortest chain of carbon atoms is the parent chain, and its name becomes the root name.
D) None of these.
A) The longest chain of carbon atoms is the parent chain, and its name becomes the root name.
B) The shortest chain of carbon atoms is the parent chain, and its name becomes the root name.
C) The longest chain of carbon atoms is the parent chain, and its name becomes the root name and the shortest chain of carbon atoms is the parent chain, and its name becomes the root name.
D) None of these.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
27
What is the molecular formula of the following compound? 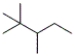
A) C8H20
B) C8H16
C) C8H17
D) C8H18

A) C8H20
B) C8H16
C) C8H17
D) C8H18

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
28
How many different substituent groups can be obtained by removing a hydrogen atom from ethane?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following are representations of the same molecule? (i) CH3CH2CH2CH2CH2CH2CH3
(ii)
 (iii)
(iii)
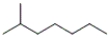
A) only (i) and (ii)
B) only (i) and (iii)
C) only (ii) and (iii)
D) none of these
(ii)
 (iii)
(iii) 
A) only (i) and (ii)
B) only (i) and (iii)
C) only (ii) and (iii)
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds are constitutional isomers? 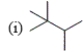
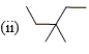
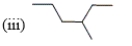
A) (i) and (ii)
B) (i) and (iii)
C) (ii) and (iii)
D) all of these

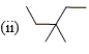

A) (i) and (ii)
B) (i) and (iii)
C) (ii) and (iii)
D) all of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following compounds among 2,3-dimethylpentane, 3-ethylpentane, and 3-methylhexane are constitutional isomers of heptane?
A) only 2,3-dimethylpentane and 3-ethylpentane
B) only 2,3-dimethylpentane and 3-methylhexane
C) only 3-ethylpentane and 3-methylhexane
D) all of these
A) only 2,3-dimethylpentane and 3-ethylpentane
B) only 2,3-dimethylpentane and 3-methylhexane
C) only 3-ethylpentane and 3-methylhexane
D) all of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a constitutional isomer of butane?
A) CH3CH2CH2CH2CH2CH2CH2CH3
B)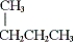
C)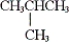
D) all of these
A) CH3CH2CH2CH2CH2CH2CH2CH3
B)
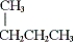
C)
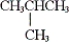
D) all of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
33
How many different substituent groups can be obtained by removing a hydrogen atom from propane?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
34
How is a isobutyl group formed?
A) by removing a hydrogen atom from the first carbon of butane
B) by removing a hydrogen atom from the second carbon of butane
C) by removing a hydrogen atom from the first carbon of 2-methylpropane
D) by removing a hydrogen atom from the second carbon of 2-methylpropane
A) by removing a hydrogen atom from the first carbon of butane
B) by removing a hydrogen atom from the second carbon of butane
C) by removing a hydrogen atom from the first carbon of 2-methylpropane
D) by removing a hydrogen atom from the second carbon of 2-methylpropane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is a constitutional isomer of hexane?
A)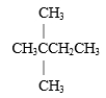
B)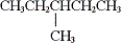
C) both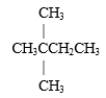 and
and
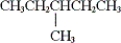
D) none of these
A)

B)
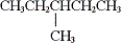
C) both
 and
and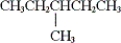
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is the correct IUPAC rule for naming an alkyl group?
A) The suffix -yl is added to the name of the parent hydrocarbon.
B) The suffix -ane is replaced with the suffix -yl.
C) The suffix -ane is replaced with the suffix -anyl.
D) The suffix -yl is inserted before the suffix -ane.
A) The suffix -yl is added to the name of the parent hydrocarbon.
B) The suffix -ane is replaced with the suffix -yl.
C) The suffix -ane is replaced with the suffix -anyl.
D) The suffix -yl is inserted before the suffix -ane.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
37
How is a butyl group formed?
A) by removing a hydrogen atom from the first carbon of butane
B) by removing a hydrogen atom from the second carbon of butane
C) by removing a hydrogen atom from the first carbon of 2-methylpropane
D) by removing a hydrogen atom from the second carbon of 2-methylpropane
A) by removing a hydrogen atom from the first carbon of butane
B) by removing a hydrogen atom from the second carbon of butane
C) by removing a hydrogen atom from the first carbon of 2-methylpropane
D) by removing a hydrogen atom from the second carbon of 2-methylpropane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
38
Of the given compounds, which compound is not a constitutional isomer of the other three compounds? 2,3-dimethylpentane, 3-ethylpentane, 2,2-dimethylbutane, and 3-methylhexane
A) 2,3-dimethylpentane
B) 3-methylhexane
C) 3-ethylpentane
D) 2,2-dimethylbutane
A) 2,3-dimethylpentane
B) 3-methylhexane
C) 3-ethylpentane
D) 2,2-dimethylbutane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following are constitutional isomers? (i) CH3CH2CH2CH2CH2CH2CH2CH3
(ii)
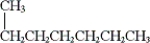 (iii)
(iii)
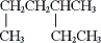
A) only (i) and (ii)
B) only (i) and (iii)
C) only (ii) and (iii)
D) none of these
(ii)
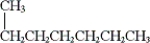 (iii)
(iii) 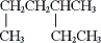
A) only (i) and (ii)
B) only (i) and (iii)
C) only (ii) and (iii)
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following are constitutional isomers? (i) CH3CH2CH2CH2CH2CH2CH2CH3
(ii)
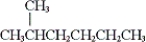 (iii)
(iii)
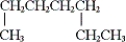
A) only (i) and (ii)
B) only (i) and (iii)
C) only (ii) and (iii)
D) none of these
(ii)
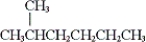 (iii)
(iii) 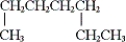
A) only (i) and (ii)
B) only (i) and (iii)
C) only (ii) and (iii)
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
41
What is the correct IUPAC name for the following compound? 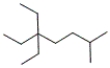
A) 3,3-diethyl-6-methylheptane
B) isododecane
C) 3,3-dimethyl-6-ethylheptane
D) 1,1,1-triethyl-4-methylpentane

A) 3,3-diethyl-6-methylheptane
B) isododecane
C) 3,3-dimethyl-6-ethylheptane
D) 1,1,1-triethyl-4-methylpentane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is a correct IUPAC name?
A) 2-ethylpropane
B) 2-ethylbutane
C) 2-ethylpentane
D) none of these
A) 2-ethylpropane
B) 2-ethylbutane
C) 2-ethylpentane
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
43
What is the IUPAC name of the given compound? 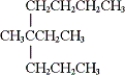
A) 4-ethyl-4-methyloctane
B) 3-methyl-3-propylheptane
C) 2-ethyl-2-propylhexane
D) none of these
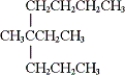
A) 4-ethyl-4-methyloctane
B) 3-methyl-3-propylheptane
C) 2-ethyl-2-propylhexane
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
44
Students were shown the structure of an alkane and were asked to write the IUPAC name of the alkane for an exam. Of the following answers that were given, which answer corresponds to the correct IUPAC name of a compound?
A) 2-ethyl-2-methylbutane
B) 2,2-dimethylbutane
C) 2,2-diethylbutane
D) none of these
A) 2-ethyl-2-methylbutane
B) 2,2-dimethylbutane
C) 2,2-diethylbutane
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
45
How is a sec-butyl group formed?
A) by removing a hydrogen atom from the first carbon of butane
B) by removing a hydrogen atom from the second carbon of butane
C) by removing a hydrogen atom from the first carbon of 2-methylpropane
D) by removing a hydrogen atom from the second carbon of 2-methylpropane
A) by removing a hydrogen atom from the first carbon of butane
B) by removing a hydrogen atom from the second carbon of butane
C) by removing a hydrogen atom from the first carbon of 2-methylpropane
D) by removing a hydrogen atom from the second carbon of 2-methylpropane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is a correct IUPAC name?
A) 1-methylpentane
B) 2-ethylpentane
C) 3-ethylpentane
D) None, they are all incorrect.
A) 1-methylpentane
B) 2-ethylpentane
C) 3-ethylpentane
D) None, they are all incorrect.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the correct line-angle structure for 3-ethylheptane?
A)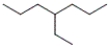
B)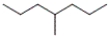
C)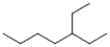
D)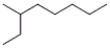
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
48
When deriving an IUPAC name, which of the following prefixes should not be ignored during the alphabetizing of substituents?
A) iso
B) di-
C) sec-
D) All of these should be ignored.
A) iso
B) di-
C) sec-
D) All of these should be ignored.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
49
A student named a compound 2,2-diethylpentane. Although this name correctly represents the connectivity of the compound, it is not a valid IUPAC name. What is the correct IUPAC name of this compound?
A) 3-ethyl-3-methylhexane
B) 3-methyl-3-propylpentane
C) 2-ethyl-2-propylbutane
D) 4,4-diethylpentane
A) 3-ethyl-3-methylhexane
B) 3-methyl-3-propylpentane
C) 2-ethyl-2-propylbutane
D) 4,4-diethylpentane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following line-angle structures is 4-isopropyloctane?
A)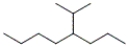
B)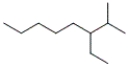
C)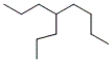
D)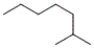
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
51
Pentane and 2-methylbutane have a third constitutional isomer called neopentane. What is the IUPAC name of neopentane?
A) 2,2-dimethylpropane
B) 2-methylisobutane
C) 2-ethylpropane
D) none of these
A) 2,2-dimethylpropane
B) 2-methylisobutane
C) 2-ethylpropane
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
52
How is a tert-butyl group formed?
A) by removing a hydrogen atom from the first carbon of butane
B) by removing a hydrogen atom from the second carbon of butane
C) by removing a hydrogen atom from the first carbon of 2-methylpropane
D) by removing a hydrogen atom from the second carbon of 2-methylpropane
A) by removing a hydrogen atom from the first carbon of butane
B) by removing a hydrogen atom from the second carbon of butane
C) by removing a hydrogen atom from the first carbon of 2-methylpropane
D) by removing a hydrogen atom from the second carbon of 2-methylpropane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
53
What is the correct IUPAC name for the following compound? 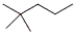
A) 2,2-dimethylhexane
B) 2,2-dimethylpentane
C) 1,1,1-trimethylbutane
D) 4,4,4-trimethylbutane

A) 2,2-dimethylhexane
B) 2,2-dimethylpentane
C) 1,1,1-trimethylbutane
D) 4,4,4-trimethylbutane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
54
What is the common name of 2-methylbutane?
A) isopentane
B) neopentane
C) 2-methylisopropane
D) none of these
A) isopentane
B) neopentane
C) 2-methylisopropane
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is a correct IUPAC name?
A) 1-methylhexane
B) 2-ethylhexane
C) 3-propylhexane
D) none of these
A) 1-methylhexane
B) 2-ethylhexane
C) 3-propylhexane
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is a correct IUPAC name?
A) 2-ethyl-3-methylpentane
B) 2,2-dimethylpentane
C) 2,2-diethylpentane
D) all of these
A) 2-ethyl-3-methylpentane
B) 2,2-dimethylpentane
C) 2,2-diethylpentane
D) all of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
57
A student named a compound 2-methyl-2-ethylbutane. Although this name correctly represents the connectivity of the compound, it is not a valid IUPAC name. What is the correct IUPAC name of this compound?
A) 2-ethyl-2-methylbutane
B) 3,3-dimethylpentane
C) 2,2-diethylpropane
D) 2,2-dimethylpentane
A) 2-ethyl-2-methylbutane
B) 3,3-dimethylpentane
C) 2,2-diethylpropane
D) 2,2-dimethylpentane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
58
What is the IUPAC name of the given compound? 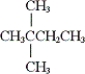
A) 2-ethyl-2-methylpropane
B) 2,2-dimethylbutane
C) 3,3-dimethylbutane
D) none of these

A) 2-ethyl-2-methylpropane
B) 2,2-dimethylbutane
C) 3,3-dimethylbutane
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
59
Which is the correct IUPAC name for the following compound? 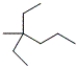
A) 2,2-diethylpentane
B) 4-ethyl-4-methylhexane
C) 4-methyl-4-ethylhexane
D) 3-ethyl-3-methylhexane

A) 2,2-diethylpentane
B) 4-ethyl-4-methylhexane
C) 4-methyl-4-ethylhexane
D) 3-ethyl-3-methylhexane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
60
When deriving an IUPAC name, which of the following prefixes should be ignored during the alphabetizing of substituents?
A) di-
B) sec-
C) tri-
D) all of these
A) di-
B) sec-
C) tri-
D) all of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
61
What is the IUPAC name of the following compound? 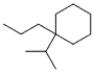
A) 1,1-dipropylcyclohexane
B) 1-isopropyl-1-propylcyclohexane
C) isopropylpropylcyclohexane
D) 1,1-isopropylbutylcyclohexane

A) 1,1-dipropylcyclohexane
B) 1-isopropyl-1-propylcyclohexane
C) isopropylpropylcyclohexane
D) 1,1-isopropylbutylcyclohexane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
62
What is the IUPAC name of the following compound? 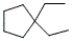
A) diethylcyclopentane
B) butylcyclopentane
C) 1,1-diethylcyclopentane
D) 1,1-diethylcyclohexane

A) diethylcyclopentane
B) butylcyclopentane
C) 1,1-diethylcyclopentane
D) 1,1-diethylcyclohexane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following compounds has isohexane as the common name?
A) CH3(CH2)4CH3
B)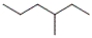
C) CH3(CH2)2CH(CH3)2
D) none of these
A) CH3(CH2)4CH3
B)

C) CH3(CH2)2CH(CH3)2
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following is the most stable conformation of cyclohexane?
A) boat
B) chair
C) envelope
D) twist
A) boat
B) chair
C) envelope
D) twist

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
65
Why do alkanes have different conformations?
A) because they have free rotation about their C-H bonds
B) because they have free rotation about their C-C bonds
C) because they have restricted rotation about their C-H bonds
D) because they have highly reactive C-C bonds
A) because they have free rotation about their C-H bonds
B) because they have free rotation about their C-C bonds
C) because they have restricted rotation about their C-H bonds
D) because they have highly reactive C-C bonds

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
66
What is the bond angle in cyclopentane?
A) 109.5°
B) 108.5°
C) 90°
D) 180°
A) 109.5°
B) 108.5°
C) 90°
D) 180°

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following hydrocarbons is a gas?
A) methane
B) hexane
C) nonane
D) pentane
A) methane
B) hexane
C) nonane
D) pentane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
68
What is the IUPAC name of the given compound? 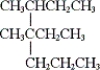
A) 2-sec-butyl-2-propylbutane
B) 4-ethyl-3,4-dimethylheptane
C) 3,4-dimethyl-3-propylhexane
D) none of these
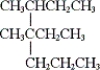
A) 2-sec-butyl-2-propylbutane
B) 4-ethyl-3,4-dimethylheptane
C) 3,4-dimethyl-3-propylhexane
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is a correct statement about the conformation given below? 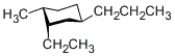
A) The ethyl and methyl groups are in the axial positions.
B) All three alkyl substituents are in equatorial positions.
C) The ethyl group is in an axial position, whereas the methyl group and propyl groups are in equatorial positions.
D) The ethyl and methyl groups are in equatorial positions, whereas the propyl group is in an axial position.

A) The ethyl and methyl groups are in the axial positions.
B) All three alkyl substituents are in equatorial positions.
C) The ethyl group is in an axial position, whereas the methyl group and propyl groups are in equatorial positions.
D) The ethyl and methyl groups are in equatorial positions, whereas the propyl group is in an axial position.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following is true of the C-H equatorial bonds in cyclohexane?
A) Each carbon atom of the ring is bonded to two hydrogen atoms at equatorial positions.
B) The C-H equatorial bonds are oriented perpendicular to the imaginary axis of the ring.
C) The C-H equatorial bonds are positioned on the same side of the ring.
D) All C-H equatorial bonds point upward.
A) Each carbon atom of the ring is bonded to two hydrogen atoms at equatorial positions.
B) The C-H equatorial bonds are oriented perpendicular to the imaginary axis of the ring.
C) The C-H equatorial bonds are positioned on the same side of the ring.
D) All C-H equatorial bonds point upward.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is true when there is a methyl group bonded to cyclohexane?
A) The methyl group will orient itself in the axial position.
B) The methyl group will orient itself in the equatorial position.
C) The methyl group first orients itself in the equatorial position then changes to the axial position.
D) The methyl group first orients itself in the axial position then changes to the equatorial position.
A) The methyl group will orient itself in the axial position.
B) The methyl group will orient itself in the equatorial position.
C) The methyl group first orients itself in the equatorial position then changes to the axial position.
D) The methyl group first orients itself in the axial position then changes to the equatorial position.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following is true of cyclobutane?
A) It is a five-membered cyclic hydrocarbon.
B) It has one carbon-carbon double bond.
C) It is a saturated cyclic hydrocarbon.
D) It is a four-membered, open-chain hydrocarbon.
A) It is a five-membered cyclic hydrocarbon.
B) It has one carbon-carbon double bond.
C) It is a saturated cyclic hydrocarbon.
D) It is a four-membered, open-chain hydrocarbon.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is the most stable conformation of cyclopentane?
A) boat
B) chair
C) envelope
D) twist
A) boat
B) chair
C) envelope
D) twist

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following is a correct IUPAC name?
A) 1-methyl-4-ethylcyclohexane
B) cis-1,4-dimethylcyclohexane
C) cis-1,1,2-trimethylcyclohexane
D) 1-methyl-1-ethylcyclobutane
A) 1-methyl-4-ethylcyclohexane
B) cis-1,4-dimethylcyclohexane
C) cis-1,1,2-trimethylcyclohexane
D) 1-methyl-1-ethylcyclobutane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following is true of the different conformations of a cycloalkane?
A) They are constitutional isomers of one another.
B) They are obtained by rotation about the carbon-carbon bonds.
C) They are obtained by the formation of new carbon-carbon bonds.
D) They are equally stable.
A) They are constitutional isomers of one another.
B) They are obtained by rotation about the carbon-carbon bonds.
C) They are obtained by the formation of new carbon-carbon bonds.
D) They are equally stable.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
76
What is the most abundant alkane present in natural gas?
A) butane
B) propane
C) ethane
D) methane
A) butane
B) propane
C) ethane
D) methane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is true of the C-H axial bonds in cyclohexane?
A) There are five C-H axial bonds in cyclohexane.
B) The C-H axial bonds are oriented parallel to the imaginary axis of the ring.
C) The C-H axial bonds are positioned on the same side of the ring.
D) All C-H axial bonds point downward.
A) There are five C-H axial bonds in cyclohexane.
B) The C-H axial bonds are oriented parallel to the imaginary axis of the ring.
C) The C-H axial bonds are positioned on the same side of the ring.
D) All C-H axial bonds point downward.

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following ring sizes of cycloalkanes are found in abundance in nature?
A) 3 and 4 carbon atom rings
B) 4 and 5 carbon atom rings
C) 5 and 6 carbon atom rings
D) 6 and 7 carbon atom rings
A) 3 and 4 carbon atom rings
B) 4 and 5 carbon atom rings
C) 5 and 6 carbon atom rings
D) 6 and 7 carbon atom rings

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
79
What is the IUPAC name of the following compound? 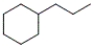
A) 2-propylcyclohexane
B) isopropylcyclohexane
C) 1-propylhexane
D) propylcyclohexane

A) 2-propylcyclohexane
B) isopropylcyclohexane
C) 1-propylhexane
D) propylcyclohexane

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck
80
What is the IUPAC name of the given compound? 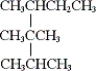
A) 2-isopropyl-2,3-dimethylpentane
B) 2-sec-butyl-2-isopropylpropane
C) 2,3,3,4-tetramethylhexane
D) none of these
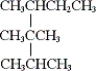
A) 2-isopropyl-2,3-dimethylpentane
B) 2-sec-butyl-2-isopropylpropane
C) 2,3,3,4-tetramethylhexane
D) none of these

Unlock Deck
Unlock for access to all 142 flashcards in this deck.
Unlock Deck
k this deck


