Deck 13: Carboxylic Acids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 13: Carboxylic Acids
1
Which reactions yield the same carboxylic acid? 
A) I, II, IV
B) I, III, IV
C) II, III, IV
D) I, II, III

A) I, II, IV
B) I, III, IV
C) II, III, IV
D) I, II, III
I, III, IV
2
What is the major product of the reaction between cinnamic acid and tryptamine in water? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
II
3
Which compounds are soluble in water? 
A) I, II, IV
B) I, III, IV
C) I, II, III
D) II, III, IV

A) I, II, IV
B) I, III, IV
C) I, II, III
D) II, III, IV
I, II, III
4
Arrange the compounds in order of increasing boiling point (lowest first). 
A) II, III, IV, I
B) I, II, III, IV
C) I, III, II, IV
D) II, III, I, IV

A) II, III, IV, I
B) I, II, III, IV
C) I, III, II, IV
D) II, III, I, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Which is the IUPAC name for the following compound? 
A) 2-oxohexanoic acid
B) 5-oxohexanoic acid
C) methyl butyroxo ketone
D) 4-ketopentanoic acid

A) 2-oxohexanoic acid
B) 5-oxohexanoic acid
C) methyl butyroxo ketone
D) 4-ketopentanoic acid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
Which compound is prepared by reaction of benzoic acid with ammonia and water? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
Arrange the compounds in order of increasing solubility in water (least soluble first). 
A) II, III, I, IV
B) IV, I, III, II
C) I, IV, II, III
D) II, III, IV, I

A) II, III, I, IV
B) IV, I, III, II
C) I, IV, II, III
D) II, III, IV, I

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
8
Which is the correct structure for Z-3-hexenedioic acid? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
9
Arrange the compounds in order of increasing boiling point (lowest first). 
A) II, I, III, IV
B) I, II, III, IV
C) III, I, II, IV
D) IV, I, II, III

A) II, I, III, IV
B) I, II, III, IV
C) III, I, II, IV
D) IV, I, II, III

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
Which structures are correctly named? 
A) I, II
B) II, III
C) III, IV
D) II, IV

A) I, II
B) II, III
C) III, IV
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
Which mixtures can be separated by treatment with aqueous NaOH? 
A) I, II
B) III, IV
C) II, IV
D) I, III

A) I, II
B) III, IV
C) II, IV
D) I, III

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
Which reactions proceed nearly to completion as written? 
A) I, II
B) III, IV
C) I, III
D) II, IV

A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
Arrange the compounds in order of increasing acidity (lowest first). 
A) III, II, I, IV
B) I, II, IV, III
C) II, III, IV, I
D) II, I, III, IV

A) III, II, I, IV
B) I, II, IV, III
C) II, III, IV, I
D) II, I, III, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
Which is the IUPAC name for the following compound? 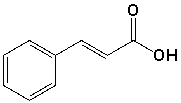
A) E-3-phenylpropenoic acid
B) Z-3-carboxy-1-phenylethene
C) E-1-phenylpropenoic acid
D) Z-benzylacrylic acid

A) E-3-phenylpropenoic acid
B) Z-3-carboxy-1-phenylethene
C) E-1-phenylpropenoic acid
D) Z-benzylacrylic acid

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
Arrange the compounds in order of increasing order of solubility in water (least soluble first). 
A) IV, III, II, I
B) III, IV, II, I
C) II, III, I, IV
D) III, II, IV, I

A) IV, III, II, I
B) III, IV, II, I
C) II, III, I, IV
D) III, II, IV, I

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
Which is the structure for potassium hydrogen oxalate? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
Arrange the compounds in order of increasing acidity (lowest first). 
A) IV, I, III, II
B) III, II, I, IV
C) II, III, I, IV
D) III, II, IV, I

A) IV, I, III, II
B) III, II, I, IV
C) II, III, I, IV
D) III, II, IV, I

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
Arrange the compounds in order of increasing acidity (lowest first). 
A) IV, I, II, III
B) III, II, IV, I
C) IV, II, I, III
D) II, I, III, IV

A) IV, I, II, III
B) III, II, IV, I
C) IV, II, I, III
D) II, I, III, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
Arrange the compounds in order of increasing order of solubility in water (least soluble first). 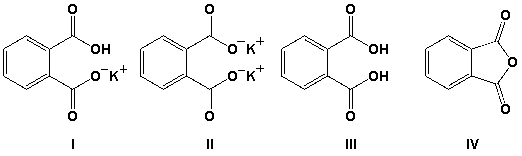
A) IV, III, I, II
B) I, III, II, IV
C) IV, II, III, I
D) I, II, III, IV

A) IV, III, I, II
B) I, III, II, IV
C) IV, II, III, I
D) I, II, III, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
Arrange the compounds in order of increasing boiling point (lowest first). 
A) IV, II, I, III
B) III, II, IV, I
C) II, III, I, IV
D) IV, III, II, I

A) IV, II, I, III
B) III, II, IV, I
C) II, III, I, IV
D) IV, III, II, I

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
Acetophenone (methyl phenyl ketone) is the product from thermal degradation of which compound? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
Which conditions are best for the transformation? 
A) LiAlH4
B) NaBH4
C) Ag(NH3)2OH / NH4OH
D) Pyridine . Cr3O4

A) LiAlH4
B) NaBH4
C) Ag(NH3)2OH / NH4OH
D) Pyridine . Cr3O4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
The major product that completes the following reaction is, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
1 Mol of benzene-1,2-dicarboxylic acid reacts with 1 mol of thionyl chloride. Which product is formed?(Hint: 2 Mols of HCl and 1 mol of SO2 are released)

A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
Which conditions are best for making the following compound? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
1 Mol of benzene-1,2-dicarboxylic acid reacts with 2 mols of thionyl chloride. Which product is formed? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
Which acid is strongest? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
Which compounds undergo thermal decarboxylation easily? 
A) I, II
B) II, III
C) III, IV
D) II, IV

A) I, II
B) II, III
C) III, IV
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
Which acid/base salts differ in their physical properties and can, in principle, be separated?
I. enantiomeric salts (racemic acid and achiral base)
II. meso-salts (meso-acid and achiral base)
III. diastereomeric salts (racemic acid and chiral base)
IV. diastereomeric salts (chiral acid and racemic base)
A) I, II
B) III, IV
C) I, III
D) II, IV
I. enantiomeric salts (racemic acid and achiral base)
II. meso-salts (meso-acid and achiral base)
III. diastereomeric salts (racemic acid and chiral base)
IV. diastereomeric salts (chiral acid and racemic base)
A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
An ether solution containing all of the following compounds was extracted first with 1 M HCl, then with 1 M NaOH, then with ether.
Which compound is extracted into the ether layer?
A) I
B) II
C) III
D) All of the above
Which compound is extracted into the ether layer?

A) I
B) II
C) III
D) All of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
The reagents that complete the following reaction are, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
Which acid is strongest? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
Which conditions will convert pentanoic acid to pentanoyl chloride?
A) HCl
B) NaCl
C) SOCl2
D) LiAlH4 followed by HCl
A) HCl
B) NaCl
C) SOCl2
D) LiAlH4 followed by HCl

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
Only the S-enantiomer of naproxen inhibits the COX-1 and COX-2 enzymes, the R-enantiomer is a strong liver toxin. Therefore, only enantiomerically pure naproxen is sold in US pharmacies. Unfortunately, the classic synthesis leads to a racemic mixture. Which base do you use to separate the two enantiomers by recrystallization? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
An ether solution containing all of the following compounds was extracted first with 1 M HCl, then with 1M NaOH. Which compound was extracted into the basic layer? 
A) I
B) II
C) III
D) All of the above

A) I
B) II
C) III
D) All of the above

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
The IUPAC name of the following structure is ___________________________. 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
The IUPAC name of the following structure is ___________________________. 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
The following compound is prepared by Fischer esterification of which combination of reagents? 
A) 1,2-dihydroxybenzene and methanol
B) salicylic acid and methanol
C) phthalic acid and methanol
D) benzoic acid and methanol

A) 1,2-dihydroxybenzene and methanol
B) salicylic acid and methanol
C) phthalic acid and methanol
D) benzoic acid and methanol

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
The amino acid tryptophan undergoes enzymatic decarboxylation to tryptamine, which is a neuromodulator in mammalian brains. What is the correct structure of tryptamine? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
The enzyme-catalyzed decarboxylation of glutamic acids leads to gamma-amino-butyric acid (GABA), which is a common neurotransmitter in the human brain. What is the chemical structure of GABA? 
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
What is the reaction product?
(Assume that sodium borohydride is added in sufficient quantities to reduce every group that can be reduced.)
(Assume that sodium borohydride is added in sufficient quantities to reduce every group that can be reduced.)


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
The reagents that complete the following reaction are, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
The product of the reaction of acetic acid and thionyl chloride is acetyl chloride.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
The product of the reaction of benzoic acid and sodium hydroxide is sodium benzoate.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
The reagents/experimental conditions that complete the following reaction are: 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
What is the reaction product? (Assume that lithium aluminum hydride is added in sufficient quantities to reduce every group that can be reduced.) 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
The product of the reaction of hexanoic acid and sodium borohydride is hexanol.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
The product of heating 4-oxopentanoic acid is butanone and carbon dioxide.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
The reagents that complete the following reaction are, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
The order of acidity of the following carboxylic acids is, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
The reagents that complete the following reaction are, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
The order of acidity of the following carboxylic acids is, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
The name of the following compound is 2,2-dibromopropanedioic acid. 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
The order of acidity of the following carboxylic acids is, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
The structure of Z-2-butendioic acid is, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
Complete the following reaction mechanism for the Fisher esterification of acetic acid. 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
The major product that completes the following reaction is, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
The major product that completes the following reaction is, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
The order of boiling points for the following compounds is, 


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


