Deck 3: Alkanes and Cycloalkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 3: Alkanes and Cycloalkanes
1
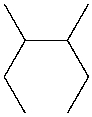
Which of the following molecules are alkanes?
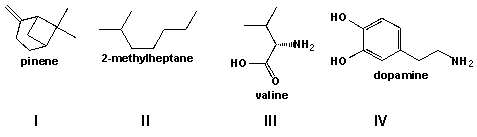
A) I and II
B) II
C) I and III
D) I and IV

A) I and II
B) II
C) I and III
D) I and IV
II
2
How many primary carbons are in THC (tetrahydrocannabinol, the main psychoactive substance found in the Cannabis plant)?
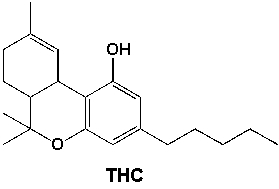
A) 2
B) 3
C) 4
D) 5

A) 2
B) 3
C) 4
D) 5
4
3
Which of the following sets are pairs of constitutional isomers?
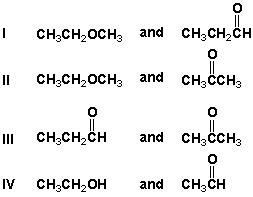
A) I
B) II
C) III
D) II and IV
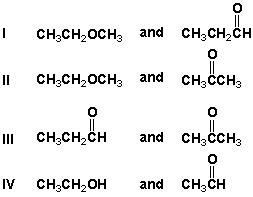
A) I
B) II
C) III
D) II and IV
III
4
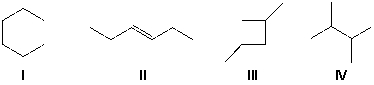
Which is the IUPAC name for the following alkane?
A) 2-ethyl-3-methylpentane
B) 3,4-dimethylhexane
C) 2,3-diethylbutane
D) 3-methyl-4-ethylpentane

A) 2-ethyl-3-methylpentane
B) 3,4-dimethylhexane
C) 2,3-diethylbutane
D) 3-methyl-4-ethylpentane

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Which is the IUPAC name for the following cycloalkane?

A) cis-1-ethyl-2-methylcyclohexane
B) trans-1-ethyl-2-methylcyclohexane
C) cis-1-methyl-2-ethylcyclohexane
D) trans-1-methyl-2-ethylcyclohexane

A) cis-1-ethyl-2-methylcyclohexane
B) trans-1-ethyl-2-methylcyclohexane
C) cis-1-methyl-2-ethylcyclohexane
D) trans-1-methyl-2-ethylcyclohexane

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
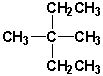
6
Which of the following molecules are constitutional isomers?
A) I and IV
B) II and III
C) I , II, and IV
D) I, II, and III

A) I and IV
B) II and III
C) I , II, and IV
D) I, II, and III

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
How many primary carbons are there in the following alkane?
A) 1
B) 2
C) 4
D) 6

A) 1
B) 2
C) 4
D) 6

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following are constitutional isomers of trans-1,2-dimethylcyclopentane?
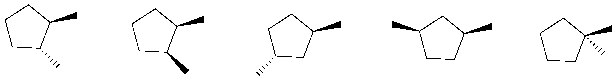
I II III IV V
A) I, III, IV
B) II, IV, V
C) II, III, V
D) III, IV, V

I II III IV V
A) I, III, IV
B) II, IV, V
C) II, III, V
D) III, IV, V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
Which is the structure for trans-1-ethyl-3-isopropylcyclohexane?
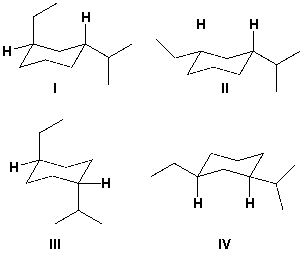
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
Which conformation of pentane is most stable?
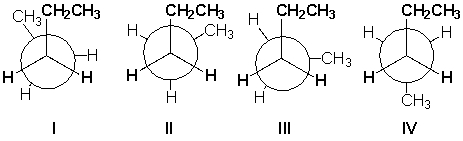
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the molecules are constitutional isomers?
A) I and II
B) I, II and III
C) I, IV, and V
D) I, II and IV

A) I and II
B) I, II and III
C) I, IV, and V
D) I, II and IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following are constitutional isomers of 4-isopropyloctane?
I. 3-ethyl-2,4,5-trimethyloctane
II. isobutylcyclohexane
III. 4-ethyl-2,2-dimethylheptane
IV. 4-ethyl-2-methyloctane
A) I, IV
B) II, III
C) I, II
D) III, IV
I. 3-ethyl-2,4,5-trimethyloctane
II. isobutylcyclohexane
III. 4-ethyl-2,2-dimethylheptane
IV. 4-ethyl-2-methyloctane
A) I, IV
B) II, III
C) I, II
D) III, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following molecules are constitutional isomers?
A) I and II
B) I, II, and III
C) I, III, and IV
D) II and III

A) I and II
B) I, II, and III
C) I, III, and IV
D) II and III

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
Which is the IUPAC name for the following cycloalkane?
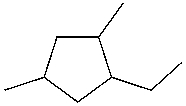
A) 2,4-dimethyl-1-ethylcyclopentane
B) 1,3-dimethyl-5-ethylcyclopentane
C) 1-ethyl-2,4-dimethylcyclopentane
D) 1-ethyl-3,5-dimethylcyclopentane

A) 2,4-dimethyl-1-ethylcyclopentane
B) 1,3-dimethyl-5-ethylcyclopentane
C) 1-ethyl-2,4-dimethylcyclopentane
D) 1-ethyl-3,5-dimethylcyclopentane

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
How many primary carbons are in cholesterol?
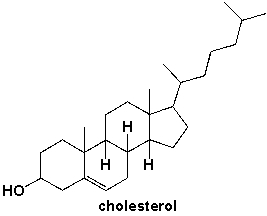
A) 2
B) 3
C) 4
D) 5

A) 2
B) 3
C) 4
D) 5

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following belong in the group of constitutional isomers for C3H6O2?
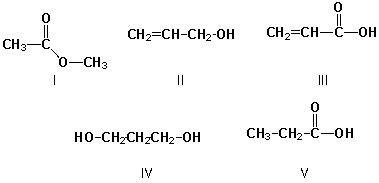
A) II, III
B) II, IV, V
C) I, II, V
D) I, V

A) II, III
B) II, IV, V
C) I, II, V
D) I, V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following molecules are constitutional isomers?
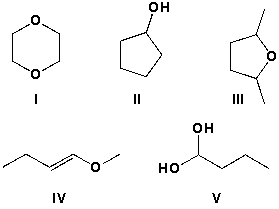
A) I and II
B) II and IV
C) II and III
D) IV and V

A) I and II
B) II and IV
C) II and III
D) IV and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
How many secondary hydrogens are there in the following alkane?
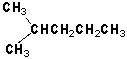
A) 1
B) 2
C) 4
D) 9
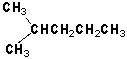
A) 1
B) 2
C) 4
D) 9

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following properties are not identical for constitutional isomers?
I. molecular formula
II. molecular weight
III. order of attachment of atoms
IV. physical properties
A) I, IV
B) II, III
C) I, II
D) III, IV
I. molecular formula
II. molecular weight
III. order of attachment of atoms
IV. physical properties
A) I, IV
B) II, III
C) I, II
D) III, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
Which pairs of molecules are constitutional isomers?
A) I, IV
B) I, II
C) II, III
D) III, IV

A) I, IV
B) I, II
C) II, III
D) III, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
In the most stable conformation of cis-1,3-dimethylcyclohexane, what positions do the methyl groups occupy?
A) axial, axial
B) equatorial, axial
C) equatorial, equatorial
D) axial, equatorial
A) axial, axial
B) equatorial, axial
C) equatorial, equatorial
D) axial, equatorial

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
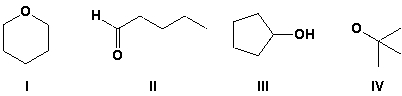
Draw a constitutional isomer of propanone (C3H6O).



Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
Which is a conformer of pentane?Which is a conformer of pentane
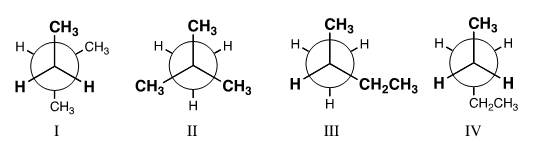
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Which molecule has the most negative heat of combustion in kcal/mole?
A) methane
B) ethane
C) propane
D) butane
A) methane
B) ethane
C) propane
D) butane

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
In the most stable conformation of trans-1,4-dimethylcyclohexane, what positions do the methyl groups occupy?
A) axial, axial
B) equatorial, axial
C) equatorial, equatorial
D) axial, equatorial
A) axial, axial
B) equatorial, axial
C) equatorial, equatorial
D) axial, equatorial

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
Which compound has the lowest boiling point?
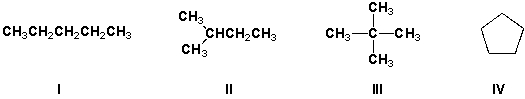
A) I
B) II
C) III
D) IV
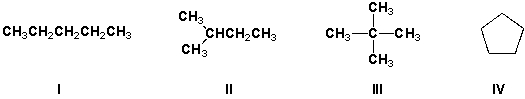
A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
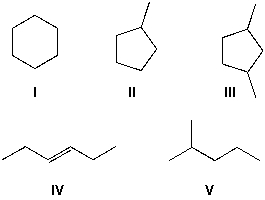
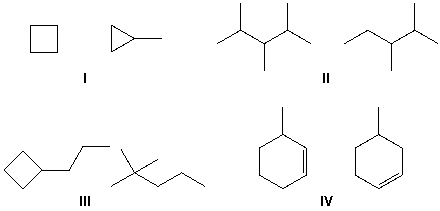
Which cycloalkanes can exist as either cis- or trans-isomers?
A) I, V
B) I, II, V
C) II, III, V
D) II, III, IV

A) I, V
B) I, II, V
C) II, III, V
D) II, III, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
What is the term for the process of forming ethene (an unsaturated hydrocarbon) from ethane (a saturated hydrocarbon)?

A) combustion
B) fractional distillation
C) thermal cracking
D) catalytic reformation

A) combustion
B) fractional distillation
C) thermal cracking
D) catalytic reformation

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
In the most stable conformation of trans-1,2-dimethylcyclohexane, what positions do the methyl groups occupy?
A) axial, axial
B) equatorial, axial
C) axial, equatorial
D) equatorial, equatorial
A) axial, axial
B) equatorial, axial
C) axial, equatorial
D) equatorial, equatorial

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Which conformation of 2-methylbutane is the most stable?Hint: Build a molecular model!
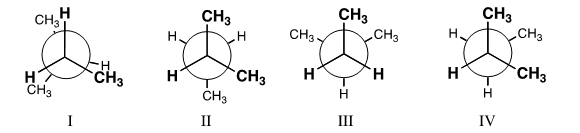
A)I
B)II
C)III
D)IV

A)I
B)II
C)III
D)IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
Which are the stoichiometric coefficients that complete the following equation?
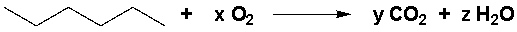
A) x = 9 ½, y = 6, z = 7
B) x = 18, y = 12, z = 14
C) x = 1, y = 1, z = 2
D) x = 6 , y = 6, z = 4
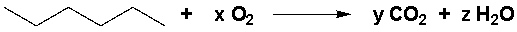
A) x = 9 ½, y = 6, z = 7
B) x = 18, y = 12, z = 14
C) x = 1, y = 1, z = 2
D) x = 6 , y = 6, z = 4

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
Which compound has the highest boiling point?
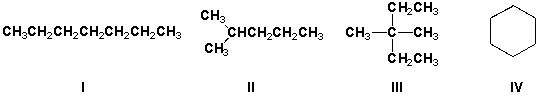
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
Which is the IUPAC name for the following compound?
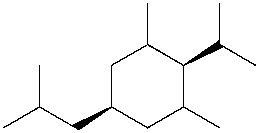
A) cis-5-isobutyl-2-isopropyl-1,3-dimethylcyclohexane
B) trans-5-isobutyl-2-isopropyl-1,3-dimethylcyclohexane
C) trans-5-isobutyl-2-isopropyl-1,3-dimethylcyclopentane
D) cis-1-isobutyl-2-isopropyl-4,5-dimethylcyclohexane

A) cis-5-isobutyl-2-isopropyl-1,3-dimethylcyclohexane
B) trans-5-isobutyl-2-isopropyl-1,3-dimethylcyclohexane
C) trans-5-isobutyl-2-isopropyl-1,3-dimethylcyclopentane
D) cis-1-isobutyl-2-isopropyl-4,5-dimethylcyclohexane

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
What is the classification of the indicated carbon?
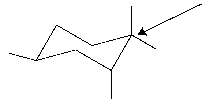


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
Which conformer of pentane is the least stable?
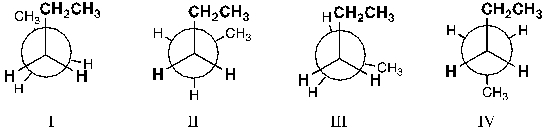
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Which conformation of 2-methylbutane is least stable?
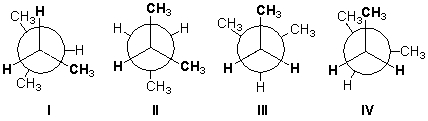
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
Which structural formulas represent cis isomers of 1,2-dimethylcyclohexane?
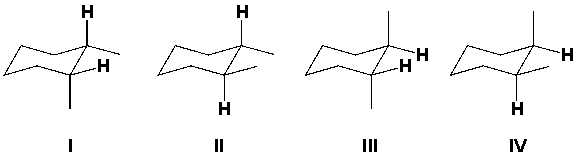
A) I, IV
B) II, III
C) III, IV
D) I, II

A) I, IV
B) II, III
C) III, IV
D) I, II

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Which is the IUPAC name for the following compound?
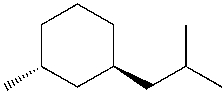
A) cis-1-isobutyl-3-methylcyclohexane
B) trans-3-isobutyl-1-methylcyclohexane
C) trans-1-ethyl-3-methylcyclohexane
D) trans-1-isobutyl-3-methylcyclohexane

A) cis-1-isobutyl-3-methylcyclohexane
B) trans-3-isobutyl-1-methylcyclohexane
C) trans-1-ethyl-3-methylcyclohexane
D) trans-1-isobutyl-3-methylcyclohexane

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
Which of these diaxial conformations has the highest energy?
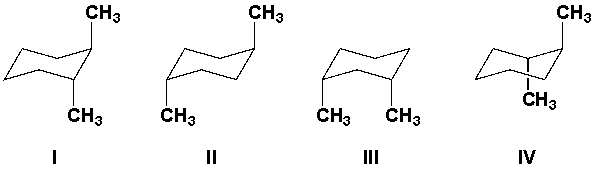
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
Which fraction of petroleum distills at the highest temperature?
A) gasoline
B) gases
C) fuel oil
D) kerosene
A) gasoline
B) gases
C) fuel oil
D) kerosene

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
The marked -CH3 group in methyl acetate is a 1o carbon.
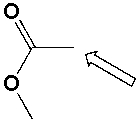


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
What is the classification of the indicated carbon?
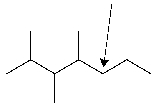


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
Alkanes all have lower densities than water.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
Propanal and propanol are constitutional isomers.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Complete the following reaction by writing the stoichiometric coefficients.
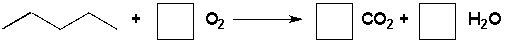
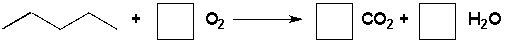

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
Pentane and cyclopentane are constitutional isomers.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
Write the name for the following molecule.
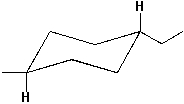


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
Complete the Newman projection for the most stable conformation of 2-methylbutane. Use the appropriate Newman projection template.
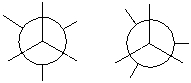


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
Complete the following reaction by writing the stoichiometric coefficients.



Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
The Newman projection below shows pentane in a gauche conformation.
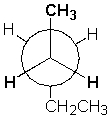


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
The Newman projection below shows 2,3-dimethylbutane in the most stable conformation.
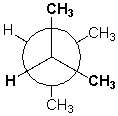


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
Complete the Newman projection for the least stable conformation of 2-methylbutane. Use the appropriate Newman projection template.
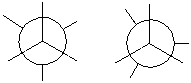


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
Write the name for the following molecule.
___________________________________________
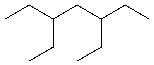
___________________________________________


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
The molecule shown has 12 primary (1°) hydrogens.
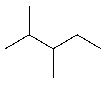


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
The following structures represent the same molecule.
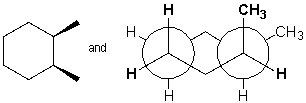


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
The marked -CH3 group in methyl acetate is a 1o carbon .
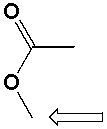


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Complete the structure by drawing in the cis-1,3-dimethyl groups.



Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
The indicated carbon is a secondary (2°) carbon.
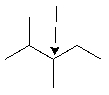


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
The following structures represent the same molecule.
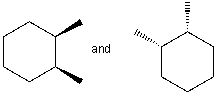


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
Complete the structure by drawing in the trans-1,4-dimethyl groups.



Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


