Deck 7: Haloalkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/58
Play
Full screen (f)
Deck 7: Haloalkanes
1
Arrange the nucleophiles in order of increasing reactivity (least reactive first).
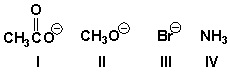
A) III, II, I, IV
B) IV, I, II, III
C) I, III, II, IV
D) III, I, IV, II
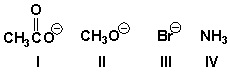
A) III, II, I, IV
B) IV, I, II, III
C) I, III, II, IV
D) III, I, IV, II
III, I, IV, II
2
Which is the best reaction condition for preparing 2-iodohexane from 1-hexene?
A) I2 / CCl4
B) HI
C) NaI
D) HIO4
A) I2 / CCl4
B) HI
C) NaI
D) HIO4
HI
3
Which of the following structures have correct common names?
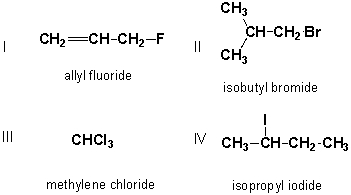
A) I, II
B) III, IV
C) I, III
D) II, IV

A) I, II
B) III, IV
C) I, III
D) II, IV
I, II
4
Which of the following haloalkanes is prepared from the following reaction?
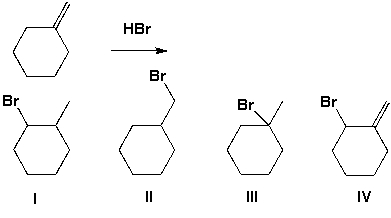
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the reactions below are most likely to be SN2 reactions?
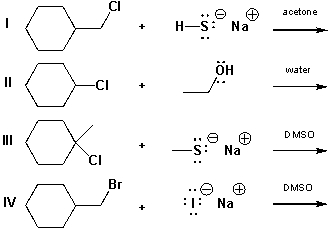
A) I, II
B) II, III
C) III, IV
D) I, IV

A) I, II
B) II, III
C) III, IV
D) I, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following structures have the correct IUPAC names?
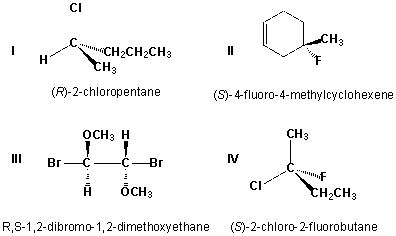
A) I, II
B) III, IV
C) I, III
D) II, IV

A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following halides is prepared from the following reaction?
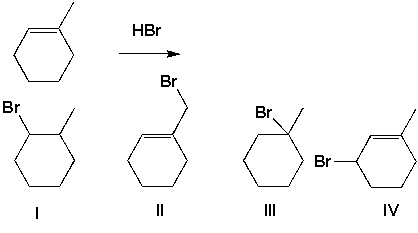
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
8
Arrange the leaving groups in order of increasing leaving group ability (least reactive first).
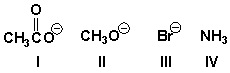
A) I, III, II, IV
B) III, I, IV, II
C) IV, II, I, III
D) IV, III, II, I
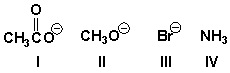
A) I, III, II, IV
B) III, I, IV, II
C) IV, II, I, III
D) IV, III, II, I

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
9
Arrange the leaving groups in order of increasing leaving group ability (least first).

A) IV, I, III, II
B) III, I, IV, II
C) II, IV, I, III
D) II, III, I, IV

A) IV, I, III, II
B) III, I, IV, II
C) II, IV, I, III
D) II, III, I, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
10
Which statements apply to an SN2 reaction?
I. The rate limiting step of the reaction involves the alkyl halide and the nucleophile.
II. The order of reactivity is methyl > 1°>2°>3°.
III. The rate limiting step of the reaction involves only the alkyl halide.
IV. There is an intermediate carbocation.
A) I, II
B) III, IV
C) I, IV
D) II, IV
I. The rate limiting step of the reaction involves the alkyl halide and the nucleophile.
II. The order of reactivity is methyl > 1°>2°>3°.
III. The rate limiting step of the reaction involves only the alkyl halide.
IV. There is an intermediate carbocation.
A) I, II
B) III, IV
C) I, IV
D) II, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
11
Which compounds are primary haloalkanes?
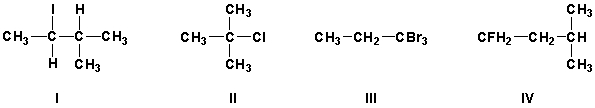
A) I, II
B) III, IV
C) II, III
D) I, IV

A) I, II
B) III, IV
C) II, III
D) I, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the reactions below are most likely to be SN1 reactions?
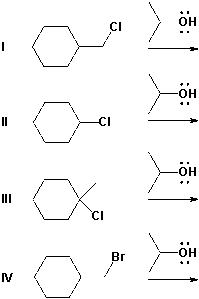
A) I, II
B) II, III
C) III, IV
D) I, IV

A) I, II
B) II, III
C) III, IV
D) I, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
13
Which compounds are tertiary haloalkanes?
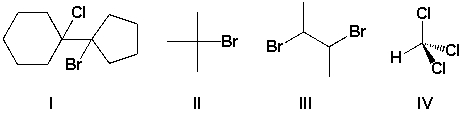
A) I and II
B) II and III
C) III and IV
D) II and IV

A) I and II
B) II and III
C) III and IV
D) II and IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
14
Which statements apply to an SN1 reaction?
I. The rate limiting step of the reaction involves the alkyl halide and the nucleophile.
II. The order of reactivity is methyl < 1°< 2° < 3°.
III. The rate limiting step of the reaction involves only the alkyl halide.
IV. There is an intermediate carbocation.
A) I, II
B) III, IV
C) I, IV
D) II, III
I. The rate limiting step of the reaction involves the alkyl halide and the nucleophile.
II. The order of reactivity is methyl < 1°< 2° < 3°.
III. The rate limiting step of the reaction involves only the alkyl halide.
IV. There is an intermediate carbocation.
A) I, II
B) III, IV
C) I, IV
D) II, III

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
15
Arrange the nucleophiles in order of increasing reactivity (least reactive first).
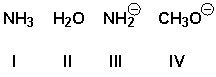
A) I, IV, II, III
B) IV, II, I, III
C) II, I, IV, III
D) IV, III, I, II
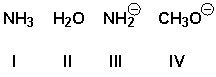
A) I, IV, II, III
B) IV, II, I, III
C) II, I, IV, III
D) IV, III, I, II

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
16
Arrange the haloalkanes in order of increasing reactivity in an SN2 reaction with KI in acetone (least first).
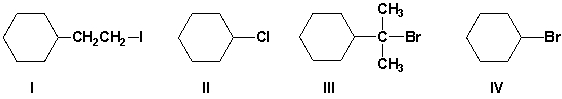
A) I, IV, III, II
B) IV, I, III, II
C) II, III, I, IV
D) III, II, IV, I

A) I, IV, III, II
B) IV, I, III, II
C) II, III, I, IV
D) III, II, IV, I

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
17
Which compounds are secondary haloalkanes?
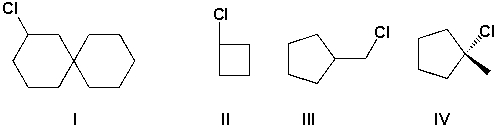
A) I and III
B) II and III
C) I and II
D) II and IV

A) I and III
B) II and III
C) I and II
D) II and IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following compounds are secondary haloalkanes?
I. Isobutyl bromide
II. 2-iodobutane
III. isopropyl fluoride
IV. neopentyl chloride
A) I, II
B) III, IV
C) II, III
D) I, IV
I. Isobutyl bromide
II. 2-iodobutane
III. isopropyl fluoride
IV. neopentyl chloride
A) I, II
B) III, IV
C) II, III
D) I, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following halides is prepared from the following reaction?
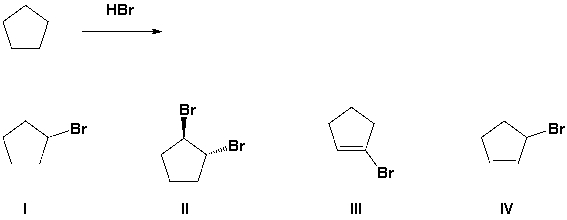
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
20
Arrange the haloalkanes in order of increasing rate of solvolysis (slowest first) in ethanol.
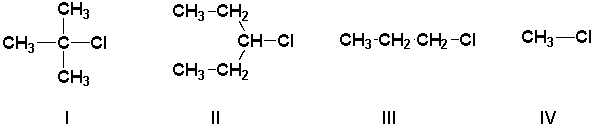
A) IV, III, II, I
B) I, II, III, IV
C) III, II, I, IV
D) II, III, I, IV
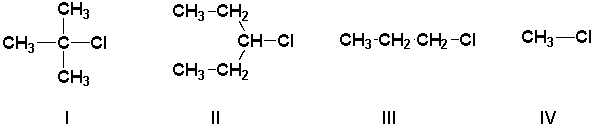
A) IV, III, II, I
B) I, II, III, IV
C) III, II, I, IV
D) II, III, I, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
21
Which reaction below should give the best yield of isopropyl methyl ether?
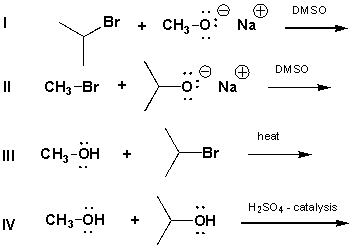
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
22
Which nucleophilic substitution reaction will proceed?
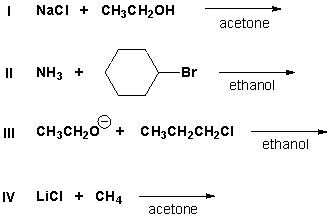
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
23
The starting material needed to complete the following reaction is,
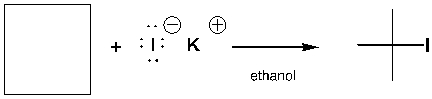


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following alkyl halides yields the product shown as the only possible product of an E2 reaction?"
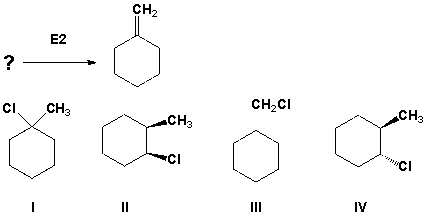
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
25
Which reaction proceeds according to an E1 mechanism?
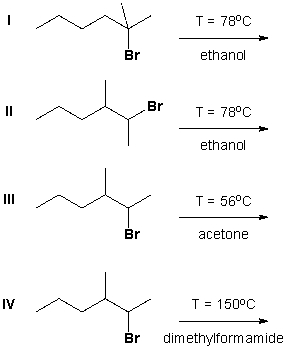
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
26
Which solvents are polar protic?
I. ethanol
II. hexane
III. DMSO
IV. water
A) III, IV
B) II, III
C) I, IV
D) I, III
I. ethanol
II. hexane
III. DMSO
IV. water
A) III, IV
B) II, III
C) I, IV
D) I, III

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
27
Which solvents are nonpolar (apolar)?
I. DMSO
II. pentane
III. methanol
IV. diethyl ether
A) II, III
B) III, IV
C) I, III
D) II, IV
I. DMSO
II. pentane
III. methanol
IV. diethyl ether
A) II, III
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
28
What products are formed in the E2 reaction shown below?
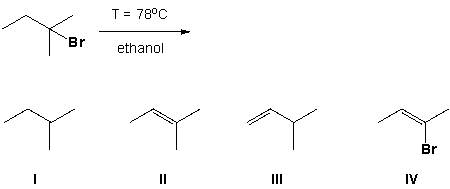
A) I and II
B) II and III
C) III and IV
D) II and IV

A) I and II
B) II and III
C) III and IV
D) II and IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
29
The major product of the following reaction is,
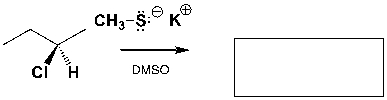


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
30
Which reactions will proceed with inversion of configuration?
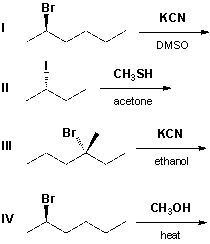
A) I, II
B) III, IV
C) II, III
D) I, IV

A) I, II
B) III, IV
C) II, III
D) I, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
31
What is the major product from an elimination reaction starting with 2-bromopentane?
A) 1-pentene
B) cis-2-pentene
C) trans-2-pentene
D) a mixture of cis and trans-2-pentene
A) 1-pentene
B) cis-2-pentene
C) trans-2-pentene
D) a mixture of cis and trans-2-pentene

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
32
What product is formed in the E1 reaction shown below?
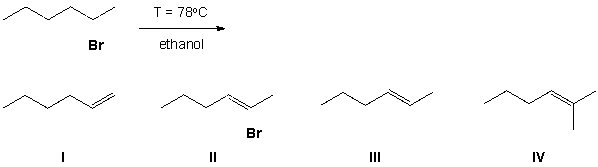
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
33
Which solvents are polar aprotic?
I. DMSO
II. H2O
III. Acetone
IV. Formic acid
A) II, III
B) III, IV
C) I, III
D) II, IV
I. DMSO
II. H2O
III. Acetone
IV. Formic acid
A) II, III
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
34
1-bromo-4-(bromomethyl)cyclohexane reacts with potassium methoxide in methanol. What is the chemical structure of the product that is formed at room temperature?
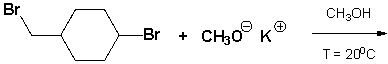
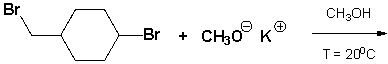

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
35
Which reaction below proceeds via an E2 mechanism?
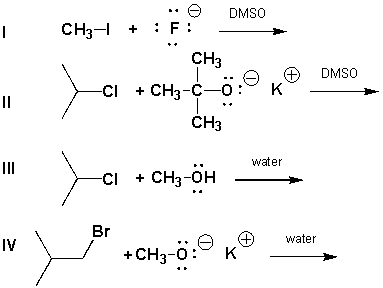
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
36
The starting material needed to complete the following reaction is,
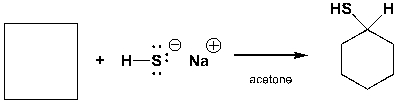
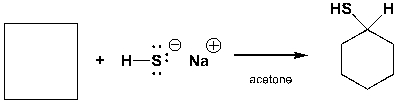

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
37
The major products of the following reaction are,
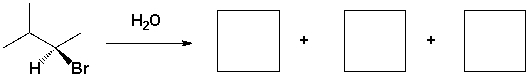


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
38
Which statement or statements is/are true for an E1 reaction?
I. The rate limiting step of the reaction involves only the alkyl halide.
II. The rate limiting step of the reaction involves the alkyl halide and the base.
III. There is an intermediate carbocation.
IV. The order of reactivity is 1°>2°>3°.
A) I, III
B) II
C) I, III, IV
D) II, IV
I. The rate limiting step of the reaction involves only the alkyl halide.
II. The rate limiting step of the reaction involves the alkyl halide and the base.
III. There is an intermediate carbocation.
IV. The order of reactivity is 1°>2°>3°.
A) I, III
B) II
C) I, III, IV
D) II, IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
39
Which reactions proceed according to an E2 mechanism?
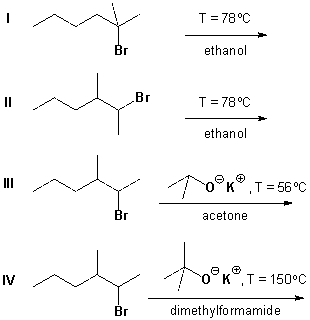
A) I and II
B) II and III
C) III and IV
D) I and IV

A) I and II
B) II and III
C) III and IV
D) I and IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the reactions below is most likely to proceed via an SN1 mechanism?
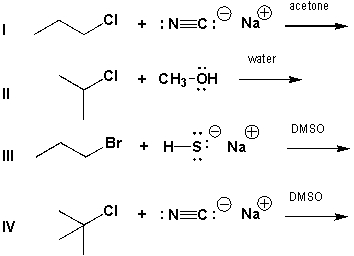
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
41
The following nucleophiles are listed in decreasing order of SN2 reactivity.
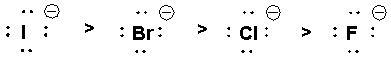
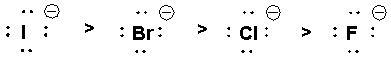

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
42
The name of the following compound is (4R, 2E) - 4-bromo-2-pentene.
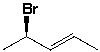
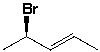

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
43
The major products of the following reaction are,
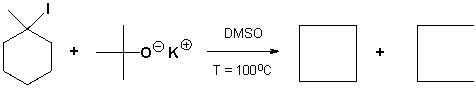


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
44
1-bromo-4-(bromomethyl)cyclohexane reacts with potassium methylthiolate in dimethylsulfoxide. Draw the chemical structure of the product that is formed at room temperature?
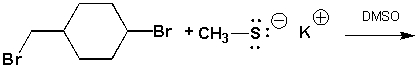
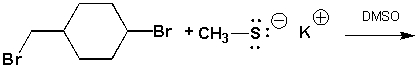

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
45
The alkyl halides below are listed in decreasing order of SN1 reactivity.
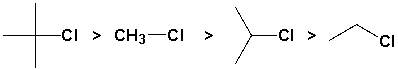
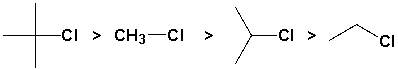

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
46
The major product of the reaction of bromocyclohexane and potassium tert-butoxide in water is an ether.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
47
The reagent needed to complete the following reaction is,
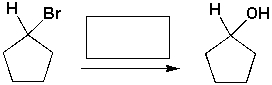


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
48
The major product of the reaction of R-2-bromobutane with potassium cyanide in acetone is S-2-methylbutanenitrile

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
49
The major product of the reaction of R-2-bromobutane with water is a racemic alcohol.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
50
1-chloro-4-(chloromethyl)cyclohexane reacts if heated until HCl leaves the molecule. What is the chemical structure of the product?
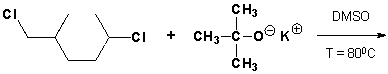
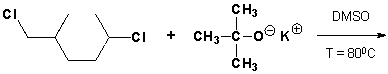

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
51
1-Chloro-4-(chloromethyl)cyclohexane reacts with two molecules of potassium-tert-butoxide in dimethylformamide. What are the two intermediate reaction products and the final reaction product formed?



Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
52
The alkyl halides below are listed in decreasing order of SN2 reactivity.
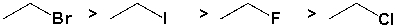

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
53
The name of the following compound is cis- (1R,2S)-1-chloro-2-bromocyclohexane.
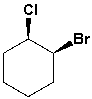


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
54
The reagent needed to complete the following reaction is,
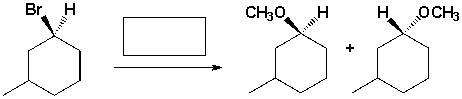


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
55
The reagent needed to complete the following reaction is,



Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
56
Polar aprotic solvents favor SN1 reactions.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
57
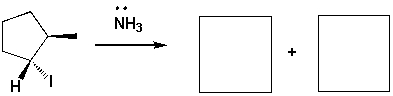
Major substitution and elimination products of the following reaction are:


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
58
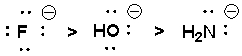
The following nucleophiles are listed in decreasing order of SN2 reactivity.


Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck


