Deck 6: Chirality: the Handedness of Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/59
Play
Full screen (f)
Deck 6: Chirality: the Handedness of Molecules
1
Rank the following substituents in order of decreasing priority (highest first).
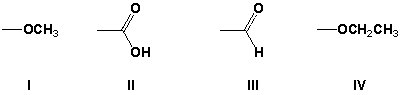
A) II, III, IV, I
B) III, IV, I, II
C) IV, III, II, I
D) IV, I, II, III
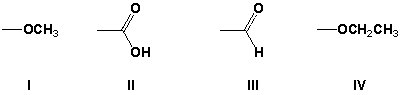
A) II, III, IV, I
B) III, IV, I, II
C) IV, III, II, I
D) IV, I, II, III
IV, I, II, III
2
Which structure is correctly named 3S,4S-3,4-dimethylhexane ?
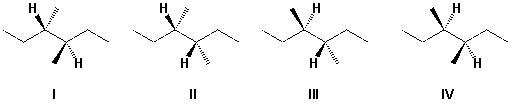
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV
IV
3
Which structures are chiral?
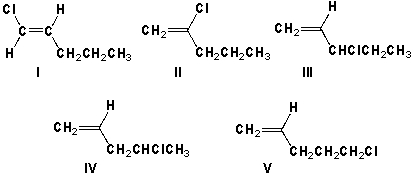
A) I, II, V
B) I, II
C) II, II, IV
D) III, IV

A) I, II, V
B) I, II
C) II, II, IV
D) III, IV
III, IV
4
What is the relationship between these two structures?
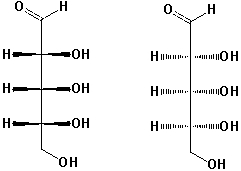
A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers

A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
5
Which forms of lactic acid are R forms?
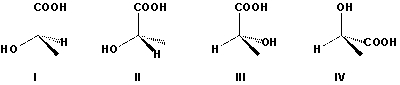
A) I, II
B) III, IV
C) II, III
D) I, IV

A) I, II
B) III, IV
C) II, III
D) I, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
6
Rank the following substituents in order of increasing priority.
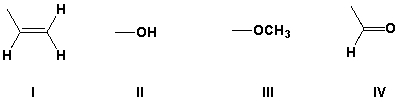
A) III, II, IV, I
B) I, IV, II, III
C) IV, I, III, II
D) I, II, III, IV
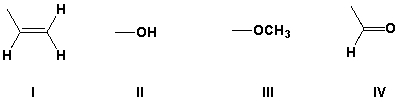
A) III, II, IV, I
B) I, IV, II, III
C) IV, I, III, II
D) I, II, III, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
7
Rank the following substituents in order of increasing priority.
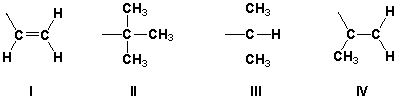
A) III, II, IV, I
B) IV, I, II, III
C) III, I, II, IV
D) IV, II, I, III

A) III, II, IV, I
B) IV, I, II, III
C) III, I, II, IV
D) IV, II, I, III

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
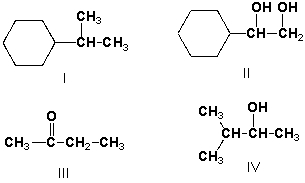
8
What is the relationship between these two structures?
A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers

A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
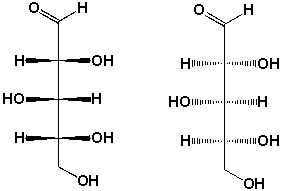
9
Which compounds contain stereocenters?
A) I, II
B) III, IV
C) I, III
D) II, IV

A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
10
Rank the following substituents in order of increasing priority.

A) I, III, II, IV
B) II, I, III, IV
C) III, I, II, IV
D) IV, III, I, II

A) I, III, II, IV
B) II, I, III, IV
C) III, I, II, IV
D) IV, III, I, II

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
11
How many stereoisomers are possible for 1,2-dichlorocyclopentane?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
12
Which pair of structures are enantiomers?
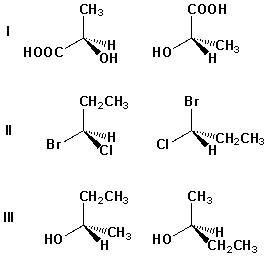
A) I, II
B) II, III
C) I, III
D) I, II, III

A) I, II
B) II, III
C) I, III
D) I, II, III

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
13
Which structures represent R-3-methylhexane?
Help: Build the structures using a molecular model.
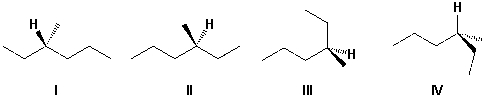
A) III, IV
B) I, II
C) I, III
D) II, IV
Help: Build the structures using a molecular model.

A) III, IV
B) I, II
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
14
What is the R,S configuration for the following structure of isocitric acid?
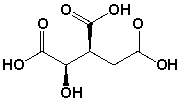
A) 2R, 3R
B) 2R, 3S
C) 2S, 3R
D) 2S, 3S

A) 2R, 3R
B) 2R, 3S
C) 2S, 3R
D) 2S, 3S

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
15
Aspartame is an artificial, non-saccharide sweetener. It is made from two amino acids: phenylalanine (methyl ester) and aspartic acid. Both aminoacids have one stereocenter. Both stereocenters have S-stereoconfiguration. Which of the two structures is aspartame?
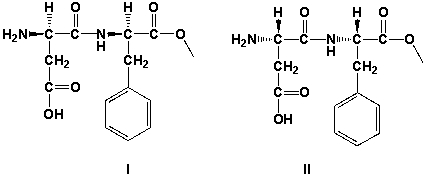
A) both structures are correct
B) I
C) II
D) Neither structure is correct.

A) both structures are correct
B) I
C) II
D) Neither structure is correct.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
16
Which compounds contain stereocenters?
I. 1-chloropentane
II. 2-chloropentane
III. 3-chloropentane
IV. 1,2-dichloropentane
A) I, II
B) III, IV
C) I, III
D) II, IV
I. 1-chloropentane
II. 2-chloropentane
III. 3-chloropentane
IV. 1,2-dichloropentane
A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
17
Which compounds have multiple stereocenters?
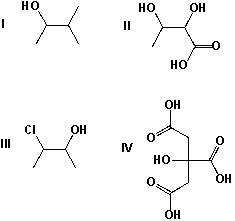
A) I, II
B) III, IV
C) II, III
D) I, III

A) I, II
B) III, IV
C) II, III
D) I, III

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
18
How many stereoisomers are possible for 2,3-butanediol?
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
19
How many stereoisomers are possible for the following structure?
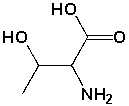
A) 1
B) 2
C) 3
D) 4

A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
20
Which compounds contain stereocenters?
I. 2-methylpentane
II. chlorocyclohexane
III. 3-methyl-2-butanol
IV. 2-hydroxypropanoic acid
A) I, II
B) III, IV
C) I, III
D) II, IV
I. 2-methylpentane
II. chlorocyclohexane
III. 3-methyl-2-butanol
IV. 2-hydroxypropanoic acid
A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
21
Which statements about stereoisomers are true? (Sec. 6.7, 6.8)
I. enantiomers and diastereomers have the same physical properties.
II. 50/50 mixtures of R and S enantiomers are called racemic mixtures.
III. meso isomers rotate the plane of plane polarized light.
IV. dextrorotatory compounds rotate plane polarized light to the right.
A) I, II
B) II, III
C) II, IV
D) III, IV
I. enantiomers and diastereomers have the same physical properties.
II. 50/50 mixtures of R and S enantiomers are called racemic mixtures.
III. meso isomers rotate the plane of plane polarized light.
IV. dextrorotatory compounds rotate plane polarized light to the right.
A) I, II
B) II, III
C) II, IV
D) III, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
22
The specific rotation of dextrorotatory tartaric acid is +12.7 degrees. A mixture of dextrorotatory and levorotatory tartaric acid has a specific rotation of +6.35 degrees. What is the optical purity of the mixture?
A) 25%
B) 33 1/3%
C) 50%
D) 75%
A) 25%
B) 33 1/3%
C) 50%
D) 75%

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
23
What is the relationship between these two structures?
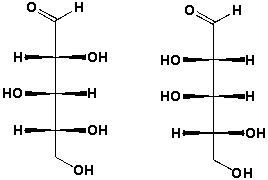
A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers

A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
24
What is the relationship between these two structures?
HINT: Build the molecular models and compare!
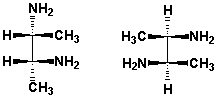
A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers
HINT: Build the molecular models and compare!
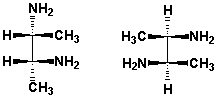
A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
25
What is the relationship between these two structures?
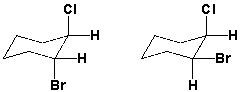
A) identical structures
B) enantiomers
C) diastereomers
D) constitutional isomers

A) identical structures
B) enantiomers
C) diastereomers
D) constitutional isomers

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
26
What is the relationship between these two structures?
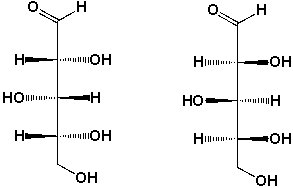
A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers

A) Identical structures
B) Enantiomers
C) Diastereomers
D) Constitutional isomers

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
27
The priority order for the following groups are (highest = 1),
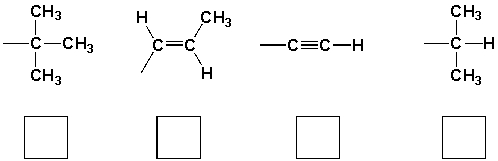


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
28
The priority order for the following groups is (highest = 1),
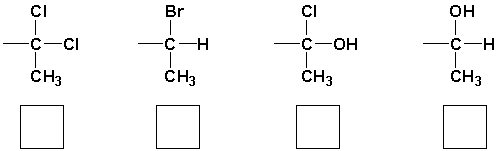


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
29
Which statement about enantiomers is false?
A) enantiomers have the same boiling and melting points.
B) enantiomers have the same chemical properties in an achiral environment.
C) enantiomers have the same atom connectivity.
D) enantiomers have the same three dimensional orientation.
A) enantiomers have the same boiling and melting points.
B) enantiomers have the same chemical properties in an achiral environment.
C) enantiomers have the same atom connectivity.
D) enantiomers have the same three dimensional orientation.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
30
Which are meso compounds?
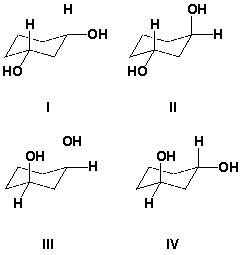
A) I and II
B) II and III
C) I and III
D) III and IV

A) I and II
B) II and III
C) I and III
D) III and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
31
Which are meso compounds?
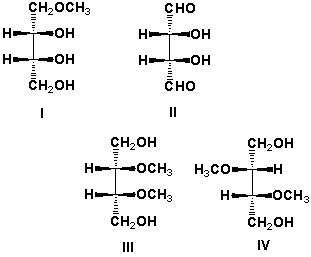
A) I, II
B) I, III
C) II, III
D) III, IV

A) I, II
B) I, III
C) II, III
D) III, IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
32
How many stereoisomers are possible for Prilosec?
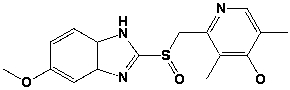
A) 0
B) 2
C) 4
D) 8

A) 0
B) 2
C) 4
D) 8

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
33
Which are meso compounds?
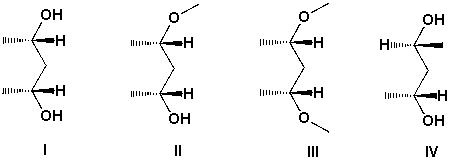
A) I and II
B) II and III
C) I and III
D) II and IV

A) I and II
B) II and III
C) I and III
D) II and IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
34
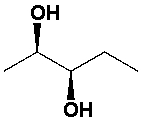
The structure of the S,S enantiomer of 2,3-butanediol is,


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
35
Tuftsin (named after Tufts University) has important functions in the human immune system. It binds to stereospecific receptors at the surface of macrophages and leukocytes, stimulating their bactericidal and tumoricidal activity. All stereocenters in this molecule have S-configurations. How many stereoisomers of tuftsin exist, which do not have the correct stereochemistry for receptor-binding?
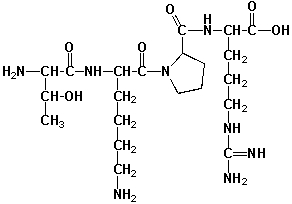
A) 7
B) 15
C) 31
D) 32

A) 7
B) 15
C) 31
D) 32

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
36
The following structure is the _____________ configuration.


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
37
How many pairs of enantiomers are possible for cholesterol?
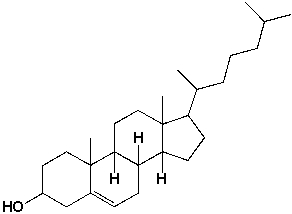
A) 16
B) 32
C) 64
D) 128

A) 16
B) 32
C) 64
D) 128

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
38
Which compound is a meso compound?
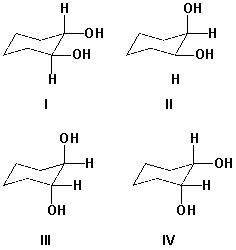
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
39
The following structure is the _____________ configuration.
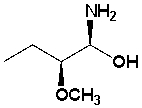


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
40
How many pairs of enantiomers are possible for cortisone acetate?
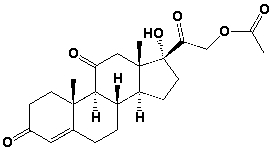
A) 32
B) 64
C) 128
D) 256

A) 32
B) 64
C) 128
D) 256

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
41
Hydrocodone, the synthetic opiate in Vicodin contains ______ stereocenters.
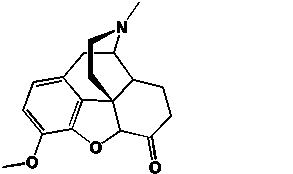


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
42
The structure of the 3S,4R enantiomer of 3-bromo-4-chlorohexane is,


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
43
The following structure contains ………..stereocenters.
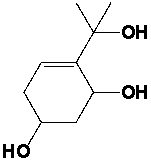


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
44
The following structure contains ___________ stereocenters.
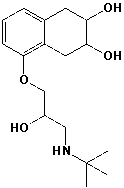


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
45
The following structures are diastereomers.
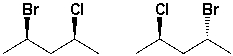


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
46
2R,4R-2-bromo-4-chloropentane and 2S,4S-2-bromo-4-chloropentane are meso compounds.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
47
Enantiomers have the same physical properties (m.p., b.p., density).

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
48
Glutathione is an antioxidant, which helps to protect our cells from oxidative damage. Assign R or S configurations to the stereocenters I and II
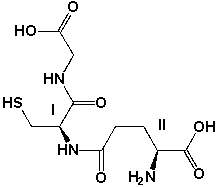


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
49
The following structure contains??____stereocenters.
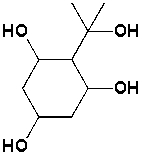


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
50
The following structures are enantiomers.
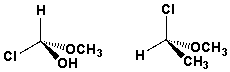


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
51
The diastereomer of 2R,3R-dichlorobutane is achiral.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
52
The name of the following structure is 2S,3S-2,3-dibromobutane.
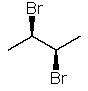


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
53
Meso compounds rotate plane polarized light the same magnitude as enantiomers, but in opposite directions.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
54
The following structure contains __________ stereocenters.
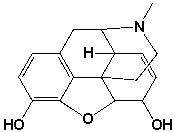


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
55
The following groups are listed in decreasing order of priority.



Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
56
The following groups are listed in decreasing order of priority.
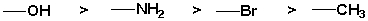
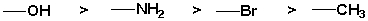

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
57
The following structures are __________________ (what type of stereoisomer?).
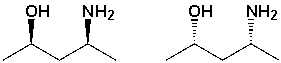
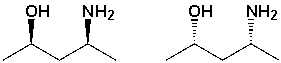

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
58
The following structures are ________________ (what type of stereoisomer).
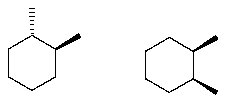


Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck
59
The diastereomers 2S,4R-2-bromo-4-chloropentane and 2S,4S-2-bromo-4-chloropentane are achiral.

Unlock Deck
Unlock for access to all 59 flashcards in this deck.
Unlock Deck
k this deck


