Deck 19: Lipids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 19: Lipids
1
The key step in the preparation of soap, saponification, is best described by which mechanism?
A) base catalyzed acyl addition
B) electrophilic addition
C) acid catalyzed acyl substitution
D) base catalyzed acyl substitution
A) base catalyzed acyl addition
B) electrophilic addition
C) acid catalyzed acyl substitution
D) base catalyzed acyl substitution
base catalyzed acyl substitution
2
Which is not a characteristic group in phospholipids?
A) phosphate esters
B) fatty acid esters
C) glycerides
D) polyamides
A) phosphate esters
B) fatty acid esters
C) glycerides
D) polyamides
polyamides
3
Fats, oils, phospholipids, prostaglandins and steroids have which properties in common?
I. oxygen functionality
II. nonpolar groups
III. rings
IV. unsaturation
A) I, II
B) III, IV
C) I, III
D) II, IV
I. oxygen functionality
II. nonpolar groups
III. rings
IV. unsaturation
A) I, II
B) III, IV
C) I, III
D) II, IV
I, II
4
Which properties are characteristic of the most abundant fatty acids found in plants and animals?
I. They contain an even number of carbon atoms, in the range 10-19.
II. The cis isomer predominates.
III. The unsaturated fatty acids have higher melting points than the corresponding saturated acids.
IV. The most abundant fatty acids are lauric, myristic and linolenic.
A) I, II
B) III, IV
C) I, III
D) II, IV
I. They contain an even number of carbon atoms, in the range 10-19.
II. The cis isomer predominates.
III. The unsaturated fatty acids have higher melting points than the corresponding saturated acids.
IV. The most abundant fatty acids are lauric, myristic and linolenic.
A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Which property of phospholipids accounts for their ability to form fluid membranes?
A) nonpolarity
B) unsaturation
C) hydrophilicity and lipophilicity
D) lipophilicity
A) nonpolarity
B) unsaturation
C) hydrophilicity and lipophilicity
D) lipophilicity

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
Which structure is a cortisone? 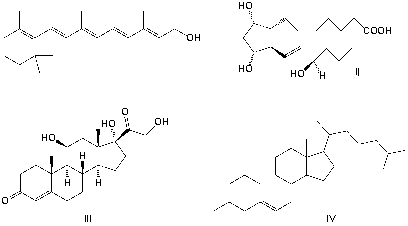
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
The fluid-mosaic model of the lipid bilayer states:
A) lipids coexist side by side as discreet units and proteins float in the bilayer, able to move along the plane of the membrane.
B) the lipids form new covalent bonds between chains and lock the proteins into position, much like a mosaic tile.
C) the lipids form a fluid-like membrane that metabolic components (the mosaic) can freely cross.
D) the lipid bilayer forms a rigid structure with channels that allow fluid to pass.
A) lipids coexist side by side as discreet units and proteins float in the bilayer, able to move along the plane of the membrane.
B) the lipids form new covalent bonds between chains and lock the proteins into position, much like a mosaic tile.
C) the lipids form a fluid-like membrane that metabolic components (the mosaic) can freely cross.
D) the lipid bilayer forms a rigid structure with channels that allow fluid to pass.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
Which are the most common fatty acids found in phospholipids?
A) palmitoleic, stearic, lauric
B) lauric, myristic, palmitic
C) palmitic, stearic, oleic
D) stearic arachidic, oleic
A) palmitoleic, stearic, lauric
B) lauric, myristic, palmitic
C) palmitic, stearic, oleic
D) stearic arachidic, oleic

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
Soap is an anionic surfactant used in conjunction with water for washing and cleaning. It is a typical example for European Medieval technology. Animal or plant fats are hydrolyzed by the strong base (NaOH in water, usually called lye), yielding alkali salts of fatty acids and glycerol. Historically, tallow (beef or mutton fat) is used for saponification. Tallow contains glycerol esters of oleic acid (47%), palmitic acid (26%) and stearic acid (14%). What are the chemical components of soap from tallow? 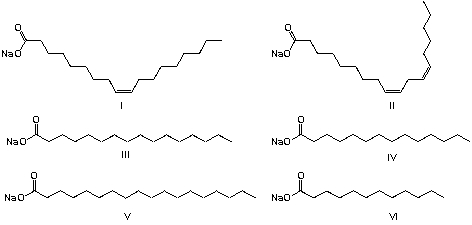
A) I, II, III
B) II, III, IV
C) I , III, V
D) III, IV, V, VI

A) I, II, III
B) II, III, IV
C) I , III, V
D) III, IV, V, VI

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
Which saturated fatty acids have the correct name?
I. CH3(CH2)10COOH - stearic acid
II. CH3(CH2)14COOH - palmitic acid
III. CH3(CH2)16COOH - lauric acid
IV. CH3(CH2)18COOH - arachidic acid
A) I, II
B) III, IV
C) I, III
D) II, IV
I. CH3(CH2)10COOH - stearic acid
II. CH3(CH2)14COOH - palmitic acid
III. CH3(CH2)16COOH - lauric acid
IV. CH3(CH2)18COOH - arachidic acid
A) I, II
B) III, IV
C) I, III
D) II, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
Which is the structure of a phosphatidyl choline? 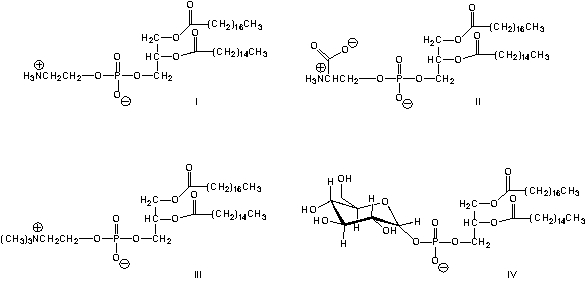
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
Deposits and films are a problem with soaps but not detergents because of which property?
A) Soaps form soluble calcium salts.
B) Detergents form more stable micelles.
C) Most detergents do not form insoluble calcium salts.
D) Soap micelles are unstable in acid.
A) Soaps form soluble calcium salts.
B) Detergents form more stable micelles.
C) Most detergents do not form insoluble calcium salts.
D) Soap micelles are unstable in acid.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Polysorbate 80 (Tween 80) is a non-ionic surfactant and emulsifier (w+x+y+z=19). A typical application is in ice cream, where it hinders the milk proteins from completely coating the fat droplets. This allows for a firmer texture, which is associated with high quality ice cream. Which of the following statements is true? 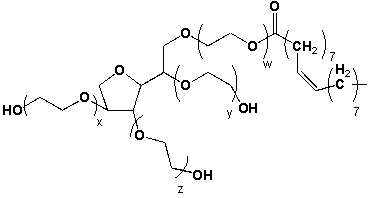 I) Polysorbate 80 is an ester.
I) Polysorbate 80 is an ester.
II) Polysorbate 80 has a hydrophilic component.
III) Polysorbate 80 has a hydrophobic component.
IV) Polysorbate 80 forms micelles above its critical micellar concentration.
A) I and II
B) II and III
C) I, II, and IV
D) all of the above
 I) Polysorbate 80 is an ester.
I) Polysorbate 80 is an ester.II) Polysorbate 80 has a hydrophilic component.
III) Polysorbate 80 has a hydrophobic component.
IV) Polysorbate 80 forms micelles above its critical micellar concentration.
A) I and II
B) II and III
C) I, II, and IV
D) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
Which reagents could be used to harden an oil to a fat?
A) NaBH4 / H2O
B) Ni / H2
C) Ag(NH3)2+ / H2O
D) Cu2+ / buffer
A) NaBH4 / H2O
B) Ni / H2
C) Ag(NH3)2+ / H2O
D) Cu2+ / buffer

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
Which physical property of triacylglycerols rich in unsaturated fatty acids is responsible for the lower melting point than the corresponding saturated compounds?
A) Hydrophobic attractions
B) Hydrophilic interactions
C) Cis-carbon-carbon double bond steric interactions
D) Hydrogen bonding
A) Hydrophobic attractions
B) Hydrophilic interactions
C) Cis-carbon-carbon double bond steric interactions
D) Hydrogen bonding

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
Today, palm oil or palm kernel oil is often used for the production of soap. The main components of palm kernel oil are glycerol esters of lauric acid (48%), myristic acid (16%) and palmitic acid (14%). What are the chemical components of soap from palm kernel oil? 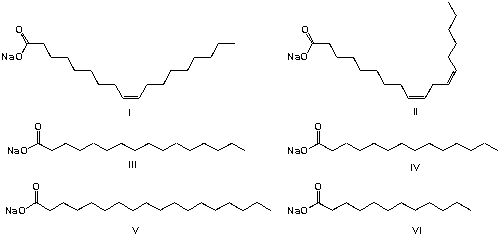
A) I, II, III
B) III, IV, V
C) III, IV, VI
D) IV, V, VI

A) I, II, III
B) III, IV, V
C) III, IV, VI
D) IV, V, VI

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
Which physical property of triacylglycerols rich in saturated fatty acids is responsible for the higher melting point than the corresponding unsaturated compounds?
A) Dispersion forces
B) Hydrophilic interactions
C) Cis-carbon-carbon double bond steric interactions
D) Hydrogen bonding
A) Dispersion forces
B) Hydrophilic interactions
C) Cis-carbon-carbon double bond steric interactions
D) Hydrogen bonding

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
Which property of phospholipids accounts for their ability to form micelles?
A) nonpolarity
B) unsaturation
C) hydrophilicity and lipophilicity
D) lipophilicity
A) nonpolarity
B) unsaturation
C) hydrophilicity and lipophilicity
D) lipophilicity

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
How many triglycerides, including stereoisomers, are possible if two fatty acids are present in the triglyceride?
A) 3
B) 4
C) 5
D) 8
A) 3
B) 4
C) 5
D) 8

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
What is the pH of a solution of an anionic detergent (typically: a soap or laundry detergent) (I), a non-ionic detergent (typically: an emulsifier in drink preparations) (II) and a cationic detergent (typically: a fabric softener) (III)?
A) I: acidic, II: neutral, III: basic
B) I: basic, II: neutral, III: acidic
C) I: basic, II: neutral, III: neutral
D) I: acidic, II: basic, III: neutral
A) I: acidic, II: neutral, III: basic
B) I: basic, II: neutral, III: acidic
C) I: basic, II: neutral, III: neutral
D) I: acidic, II: basic, III: neutral

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
Which is the best description of the first step (A) of the biosynthesis of prostaglandin?
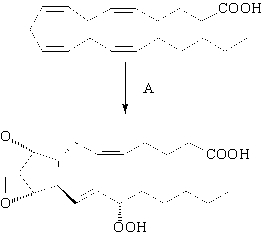
A) hydration
B) reduction
C) oxidation
D) Claisen condensation

A) hydration
B) reduction
C) oxidation
D) Claisen condensation

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
What do all prostaglandins have in common?
I. Prostaglandins are only biosynthesized in males.
II. All prostaglandins feature 19 carbon atoms.
III. All prostaglandins are alkenes.
IV. All prostaglandins feature one carbocylic ring, but the size of the ring may vary.
A) I and III
B) II and IV
C) I and IV
D) II and III
I. Prostaglandins are only biosynthesized in males.
II. All prostaglandins feature 19 carbon atoms.
III. All prostaglandins are alkenes.
IV. All prostaglandins feature one carbocylic ring, but the size of the ring may vary.
A) I and III
B) II and IV
C) I and IV
D) II and III

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
Order the four vitamins according to increasing fat-solubility (most hydrophilic first): 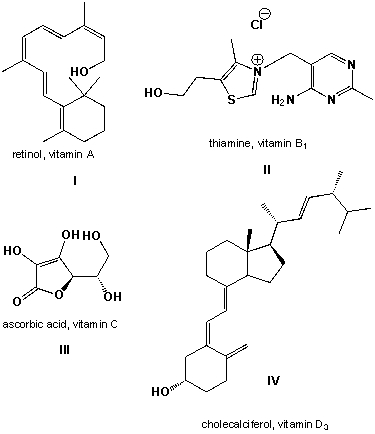
A) II, III, I, IV
B) IV, II, III. I
C) III, II. I. IV
D) I, II, III, IV

A) II, III, I, IV
B) IV, II, III. I
C) III, II. I. IV
D) I, II, III, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Complete the reaction below by providing the missing products. 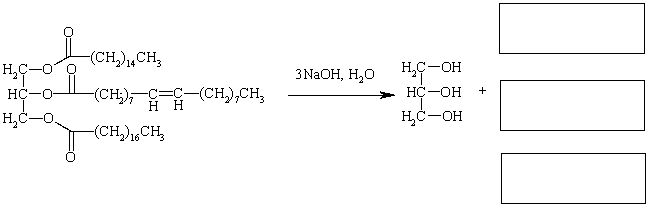


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
The melting point order of the following fatty acids is (lowest to highest),
lauric (12:0) palmitic (16:0) palmitoleic (16:1) oleic (18:1)
_______ _______ _______ ______
lauric (12:0) palmitic (16:0) palmitoleic (16:1) oleic (18:1)
_______ _______ _______ ______

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
Complete the structure of the alkylbenzene sulfonate detergent below. 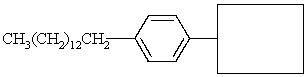


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
Fluoxymesterone is an anabolic steroid with strong androgenic properties, which has been used for the treatment of hormone-sensitive breast tumors in women. It is also misused as an aggression enhancer in boxing and martial arts competitions. Since the half-life of this hormone in the human body is more than 9h, it can be easily detected in a doping sample taken after the competition. Compare the in-vivo physical properties of both hormones. Which statements below are correct? 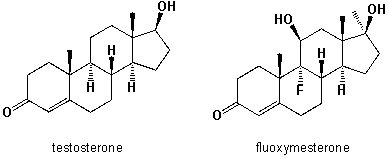 I) The exchange of a hydrogen atom vs. a fluorine atom does not significantly change the water-solubility, because the C-F bond is short and an organically bound fluorine-atom is almost as small as a carbon-bonded hydrogen atom.
I) The exchange of a hydrogen atom vs. a fluorine atom does not significantly change the water-solubility, because the C-F bond is short and an organically bound fluorine-atom is almost as small as a carbon-bonded hydrogen atom.
II) The exchange of a hydrogen vs. a fluorine atom does significantly change the water-solubility, because the C-F bond is non-polar covalent, whereas C-H is polar covalent.
III) The introduction of a second hydroxy-group at the C-ring of fluoxymesterone increases the water-solubility.
IV) Fluoxymesterone is less water soluble than testosterone, because it possesses 19 carbon atoms, compared to 19 of testosterone.
A) I and II
B) II and III
C) I and III
D) I, III, and IV
 I) The exchange of a hydrogen atom vs. a fluorine atom does not significantly change the water-solubility, because the C-F bond is short and an organically bound fluorine-atom is almost as small as a carbon-bonded hydrogen atom.
I) The exchange of a hydrogen atom vs. a fluorine atom does not significantly change the water-solubility, because the C-F bond is short and an organically bound fluorine-atom is almost as small as a carbon-bonded hydrogen atom.II) The exchange of a hydrogen vs. a fluorine atom does significantly change the water-solubility, because the C-F bond is non-polar covalent, whereas C-H is polar covalent.
III) The introduction of a second hydroxy-group at the C-ring of fluoxymesterone increases the water-solubility.
IV) Fluoxymesterone is less water soluble than testosterone, because it possesses 19 carbon atoms, compared to 19 of testosterone.
A) I and II
B) II and III
C) I and III
D) I, III, and IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
Which is a prostaglandin? 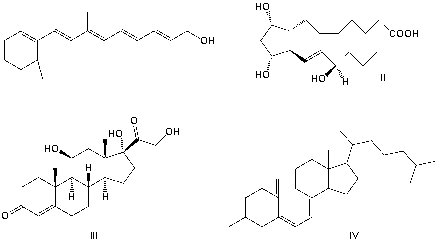
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Which vitamins are soluble in lipids? 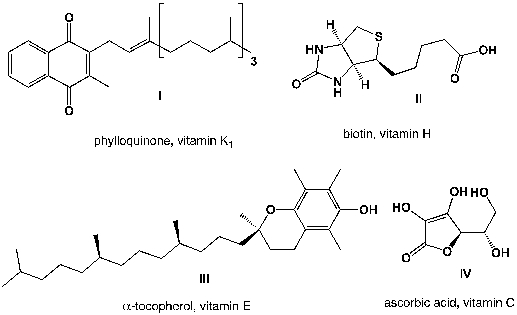
A) II and III
B) III and IV
C) I and III
D) II and IV

A) II and III
B) III and IV
C) I and III
D) II and IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Which statements about steroids are false?
I. The fusion of all rings is cis.
II. Sex hormones, adrenocorticoid hormones, bile acids and vitamin D are derived from
Cholesterol.
III. The biosynthesis of cholesterol produces several isomers.
IV. Steroids are tetracyclic ring systems
A) I, III
B) II, IV
C) I, II
D) III, IV
I. The fusion of all rings is cis.
II. Sex hormones, adrenocorticoid hormones, bile acids and vitamin D are derived from
Cholesterol.
III. The biosynthesis of cholesterol produces several isomers.
IV. Steroids are tetracyclic ring systems
A) I, III
B) II, IV
C) I, II
D) III, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
Lauramide diethanolamine (lauramide DEA, the main component of cocamide DEA) is prepared by reacting lauric acid from coconut oil with diethanolamine. It is a viscous liquid and a typical foaming agent in shampoos and bath products. Complete the following sequence of reactions. 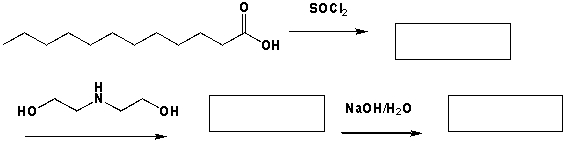


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
Which is the best description of the second step (B) of the biosynthesis of prostaglandins?
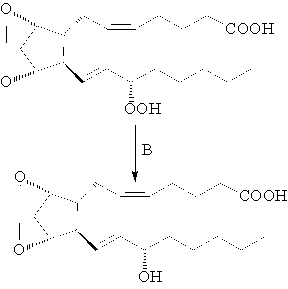
A) hydration
B) reduction
C) oxidation
D) Claisen condensation

A) hydration
B) reduction
C) oxidation
D) Claisen condensation

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
Which natural products have structures derived from cholesterol?
I. vitamin A
II. vitamin D
III. cholic acid
IV. cortisone
V. squalene
A) I, II, III
B) II, III, IV
C) III, IV, V
D) I, III, V
I. vitamin A
II. vitamin D
III. cholic acid
IV. cortisone
V. squalene
A) I, II, III
B) II, III, IV
C) III, IV, V
D) I, III, V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
Which structure is vitamin A? 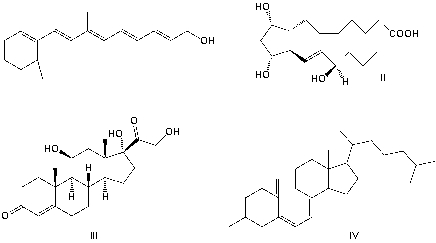
A) I
B) II
C) III
D) IV

A) I
B) II
C) III
D) IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
Complete the structure of the phospholipid below. 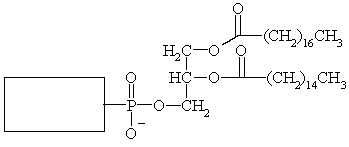


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Which is the number of stereocenters in methandrostenolone? 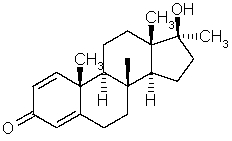
A) 4
B) 6
C) 8
D) 10

A) 4
B) 6
C) 8
D) 10

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
The lower melting point of unsaturated fatty acids compared to saturated fatty acids is due to ______________________________ differences.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Which common structural feature of vitamin E and vitamin K1 best accounts for the greater solubility of these molecules in organic solvents than water? 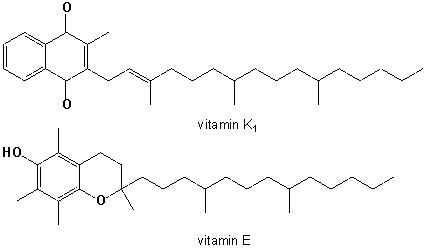
A) aromatic rings
B) oxygen atoms
C) 4 isoprene units
D) a quinone / hydroquinone units

A) aromatic rings
B) oxygen atoms
C) 4 isoprene units
D) a quinone / hydroquinone units

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
Which statements about vitamins are true?
I. Vitamins A, D, E and K are fat soluble.
II. Vitamins A, D, E and K are derived from cholesterol.
III. Vitamins A, D, E and K each contain 4 isoprene units.
IV. Vitamins A, D, E and K have distinct physiological activities.
A) II, III
B) III, IV
C) I, III
D) I, IV
I. Vitamins A, D, E and K are fat soluble.
II. Vitamins A, D, E and K are derived from cholesterol.
III. Vitamins A, D, E and K each contain 4 isoprene units.
IV. Vitamins A, D, E and K have distinct physiological activities.
A) II, III
B) III, IV
C) I, III
D) I, IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
Shown are cholesterol, estradiol, testosterone (see N13) and progesterone, which is the female pregnancy hormone. Cholesterol and all three hormones are present in both, males and females, albeit in different concentration. Their biosynthesis follows one common scheme. Based on the structures shown below, which is the hormone that is formed as an intermediate during the biosynthesis of the other two hormones? 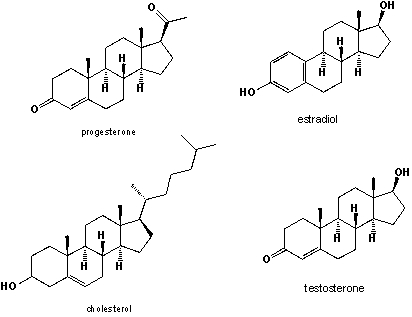
A) cholesterol
B) progesterone
C) estradiol
D) testosterone

A) cholesterol
B) progesterone
C) estradiol
D) testosterone

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
Human fat has more unsaturated fatty acids than plant fats.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
Bile acids are synthesized in the liver by cytochrome p450-mediated oxidation of cholesterol. They are often conjugated with the amino acid glycine and stored in the gallbladder. What are the differences in molecular structure between cholesterol and the cholic-acid conjugate shown below? 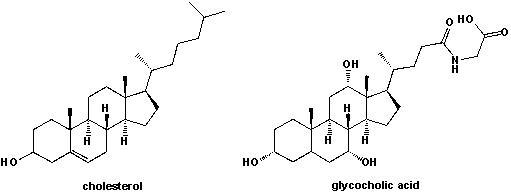


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
What are the main structural differences between molecules of estradiol (the predominant sex hormone in females, albeit present in males at lower levels) and testosterone (the principal male sex hormone and an anabolic steroid albeit it is also synthesized by females)? 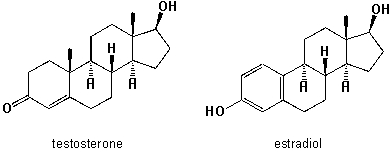


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
Aldosterone is an androgen.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Stearic acid is an unsaturated fatty acid.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
Vitamin A precursor -carotene belongs to the _____________ class of compounds and contains ________ isoprene units.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
Prostaglandin is a member of the _________________ class of compounds, which are synthesized from ____________________.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
The forces that drive micelle and lipid bilayer formation are the same.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
The forces which drive bilayer formation by phospholipids are __________________ and ________________.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
Vitamin D precursors have the cholesterol ring system.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
Why do ectothermic (cold-blooded) animals have significantly higher amounts of unsaturated fatty acids in their fatty acid signatures than endothermic (warm blooded) animals?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
There are several stereoisomers of cholesterol found in living systems.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
Dipalmitoylphosphatidylcholine (DPPC) is a phospholipid that is the major constituent of pulmonary surfactant. It is also used as a model phospholipid in numerous studies of liposomes, lipid bilayers and model biological membranes. Complete the following sequence of reactions. 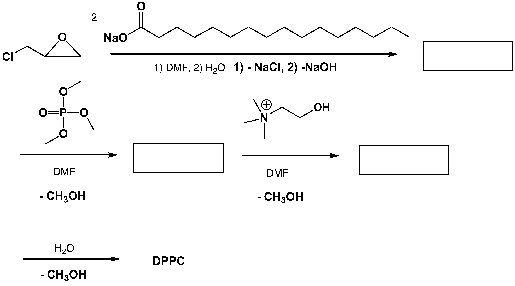


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
Vitamins A, D, E, and K are included in the lipid class because they are _______ soluble.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
Soaps and detergents form micelles when added to water.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
17- -Ethinyl-estradiol is an orally bio-active estrogen used in almost all modern formulations of contraceptive pills. It is one of the most commonly used medications. Complete the following synthetic scheme: 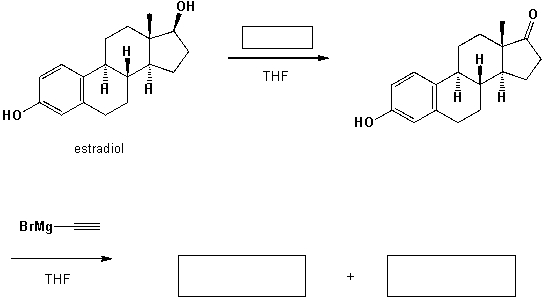


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Prostaglandins are involved with the inflammatory response.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
Vitamin E is required for blood clotting.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
Androsterone has ________ stereocenters and ____________ possible stereoisomers. 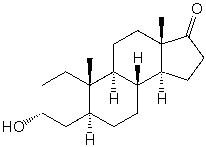


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
Soap micelles form in water by aggregating the negatively charged carboxylate groups toward the inside and the lipophilic carbon chains toward the outside of the micelle.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


