Deck 14: Organic Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/107
Play
Full screen (f)
Deck 14: Organic Compounds
1
Which of the following stick structures could describe pentane (C5H₁₂)? 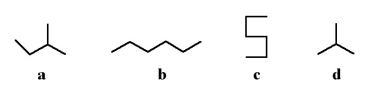
A)a
B)b
C)c
D)d
E)none of the above

A)a
B)b
C)c
D)d
E)none of the above
A
2
Which of the following molecules could be a different conformation of the underlined molecule below? 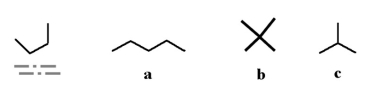
A)a
B)b
C)c
D)all of the above
E)none of the above

A)a
B)b
C)c
D)all of the above
E)none of the above
E
3
How does fractional distillation work?
A)It takes advantage of the different boiling points of molecules to separate them.
B)It takes advantage of the different weight of molecules to separate them.
C)It uses the fraction of carbon in the isomers to separate the molecules.
D)It takes advantage of the different melting points to separate molecules.
E)none of the above
A)It takes advantage of the different boiling points of molecules to separate them.
B)It takes advantage of the different weight of molecules to separate them.
C)It uses the fraction of carbon in the isomers to separate the molecules.
D)It takes advantage of the different melting points to separate molecules.
E)none of the above
A
4
Where would you expect to isolate molecules in a fractionating tower that have very few interatomic attractions?
A)near the bottom
B)near the top
C)near the middle
D)Fractional distillation takes advantage of molecular weight.
E)Fractional distillation takes advantage of the number of isomers.
A)near the bottom
B)near the top
C)near the middle
D)Fractional distillation takes advantage of molecular weight.
E)Fractional distillation takes advantage of the number of isomers.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
5
What would the formula be for the following stick model? 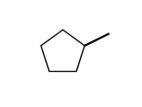
A)C₆H₁₂
B)C5H5
C)C₆H10
D)C5H10
E)none of the above

A)C₆H₁₂
B)C5H5
C)C₆H10
D)C5H10
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
6
What would the formula be for the following stick model? 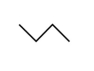
A)C4H10
B)C₃H₃
C)C₃H6
D)C₃H₈
E)C4H8

A)C4H10
B)C₃H₃
C)C₃H6
D)C₃H₈
E)C4H8

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
7
What determines the chemical and physical properties of hydrocarbons?
A)the way the atoms are connected together
B)the number of carbon and hydrogens
C)the elements it is composed of
D)the number of oxygen
E)both A and B
A)the way the atoms are connected together
B)the number of carbon and hydrogens
C)the elements it is composed of
D)the number of oxygen
E)both A and B

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following molecules could be a different conformation of the underlined molecule below? 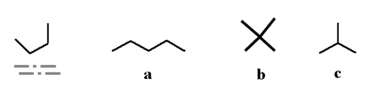
A)a
B)b
C)c
D)all of the above
E)none of the above

A)a
B)b
C)c
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following molecules is not a straight-chain hydrocarbon? 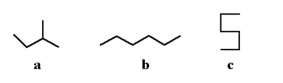
A)a
B)b
C)c
D)All of the above are straight-chain hydrocarbons.
E)None of the above are straight-chain hydrocarbons.

A)a
B)b
C)c
D)All of the above are straight-chain hydrocarbons.
E)None of the above are straight-chain hydrocarbons.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following molecules would probably be isolated closest to the top of a fractionating tower at a refinery?
A)C4H10
B)C8H18
C)C10H₂2
D)C20H₄2
E)C40H82
A)C4H10
B)C8H18
C)C10H₂2
D)C20H₄2
E)C40H82

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements best describes the concept of structural conformation?
A)rearranging the shape of a molecule by rotating around bonds
B)rearranging the shape of a molecule by changing the arrangement of bonds
C)changing the structure by adding or subtracting bonds
D)arranging the atom into the best conformation for the given structure
E)changing the conformation to generate a new molecule with different chemical properties
A)rearranging the shape of a molecule by rotating around bonds
B)rearranging the shape of a molecule by changing the arrangement of bonds
C)changing the structure by adding or subtracting bonds
D)arranging the atom into the best conformation for the given structure
E)changing the conformation to generate a new molecule with different chemical properties

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
12
Where would you expect to isolate molecules in a fractionating tower that have a large number of interatomic forces?
A)near the bottom
B)near the top
C)near the middle
D)Fractional distillation takes advantage of molecular weight.
E)Fractional distillation takes advantage of the number of isomers.
A)near the bottom
B)near the top
C)near the middle
D)Fractional distillation takes advantage of molecular weight.
E)Fractional distillation takes advantage of the number of isomers.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following two stick structures are structural isomers? 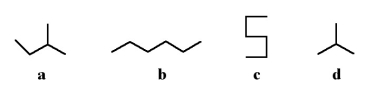
A)a and b
B)a and c
C)b and c
D)b and d
E)none of the above

A)a and b
B)a and c
C)b and c
D)b and d
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following molecules is a branched hydrocarbon? 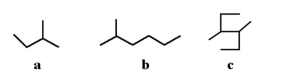
A)a
B)b
C)c
D)All of the above are branched hydrocarbons.
E)only a and b

A)a
B)b
C)c
D)All of the above are branched hydrocarbons.
E)only a and b

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
15
What is a hydrocarbon?
A)It is a molecule composed of carbon and hydrogen only.
B)It is a wet carbon atom
C)It is any organic molecule.
D)It is a molecule derived from hydrogen synthesis.
E)none of the above
A)It is a molecule composed of carbon and hydrogen only.
B)It is a wet carbon atom
C)It is any organic molecule.
D)It is a molecule derived from hydrogen synthesis.
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following two stick structures are different conformations of the same molecule? 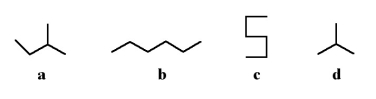
A)a and c
B)b and c
C)a and d
D)b and d
E)none of the above

A)a and c
B)b and c
C)a and d
D)b and d
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following molecules could be a structural isomer for the underlined molecule below? 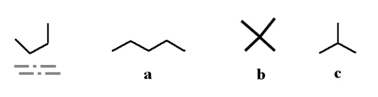
A)a
B)b
C)c
D)all of the above
E)none of the above

A)a
B)b
C)c
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules would probably be isolated closest to the bottom of a fractionating tower at a refinery?
A)C4H10
B)C8H18
C)C10H₂2
D)C20H₄2
E)C40H82
A)C4H10
B)C8H18
C)C10H₂2
D)C20H₄2
E)C40H82

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
19
What is an organic compound?
A)any molecule that is made mostly of carbon with no metals in it
B)compound grown with organic farming methods
C)any compound isolated from living organisms
D)any compound where there are metals bound to other types of atoms
E)anything that is a salt obtained from organic creatures
A)any molecule that is made mostly of carbon with no metals in it
B)compound grown with organic farming methods
C)any compound isolated from living organisms
D)any compound where there are metals bound to other types of atoms
E)anything that is a salt obtained from organic creatures

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the simplest possible hydrocarbon?
A)CH₄
B)H₂
C)HC CH
CH
D)H₂C CH₂
CH₂
E)C2H6
A)CH₄
B)H₂
C)HC
 CH
CHD)H₂C
 CH₂
CH₂E)C2H6

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements best describes the effect of heteroatoms on hydrocarbons?
A)Heteroatoms greatly alter the chemical and physical properties of a hydrocarbon.
B)Heteroatoms have little effect on the chemical and physical properties of a hydrocarbon.
C)Heteroatoms have very similar properties to hydrocarbons.
D)The properties of the heteroatom compounds depend only on the atom, not how it is bonded to the hydrocarbon.
E)none of the above
A)Heteroatoms greatly alter the chemical and physical properties of a hydrocarbon.
B)Heteroatoms have little effect on the chemical and physical properties of a hydrocarbon.
C)Heteroatoms have very similar properties to hydrocarbons.
D)The properties of the heteroatom compounds depend only on the atom, not how it is bonded to the hydrocarbon.
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
22
What is indicated by a gasoline's octane rating?
A)the degree of branching in the gasoline hydrocarbons
B)the degree at which the compound ignites
C)the percentage of octane in the mixture
D)a measure of how many degrees it takes to ignite a mixture of octane
E)none of the above
A)the degree of branching in the gasoline hydrocarbons
B)the degree at which the compound ignites
C)the percentage of octane in the mixture
D)a measure of how many degrees it takes to ignite a mixture of octane
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
23
In a fractionating tower, the crude oil vapors pass from a pipe still into the column. Tar and lubricating stock are the first components to be pulled off at the bottom. Nearer the top kerosene is pulled off followed by gasoline and finally natural gas at the very top. From this information, which has a higher boiling point, gasoline or kerosine?
A)Gasoline has the higher boiling point.
B)Kerosene has the higher boiling point.
C)Their boiling points are the same, but kerosene has the greater density.
D)Fractional distillation components are pulled off based on molecular weight, so it is not possible to know which has the higher boiling point from the information given.
A)Gasoline has the higher boiling point.
B)Kerosene has the higher boiling point.
C)Their boiling points are the same, but kerosene has the greater density.
D)Fractional distillation components are pulled off based on molecular weight, so it is not possible to know which has the higher boiling point from the information given.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the above sections of this molecule would be considered an unsaturated portion of the molecule?
A)a
B)b
C)c
D)a and b
E)all of the above
A)a
B)b
C)c
D)a and b
E)all of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
25
Why does the melting point of hydrocarbons increase as the number of carbon atoms per molecule increases?
A)An increase in the number of carbon atoms per molecules also means an increase in the density of the hydrocarbon.
B)because of greater induced dipole-induced dipole molecular attractions
C)Larger hydrocarbon chains tend to be branched.
D)The molecular mass also increases.
A)An increase in the number of carbon atoms per molecules also means an increase in the density of the hydrocarbon.
B)because of greater induced dipole-induced dipole molecular attractions
C)Larger hydrocarbon chains tend to be branched.
D)The molecular mass also increases.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is not a heteroatom?
A)O
B)N
C)P
D)A and B
E)All of the above are heteroatoms.
A)O
B)N
C)P
D)A and B
E)All of the above are heteroatoms.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
27
What is the chemical formula for the following structure? 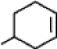
A)C6H10
B)C6H9
C)C7H12
D)C7H11

A)C6H10
B)C6H9
C)C7H12
D)C7H11

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is the hallmark of a saturated hydrocarbon?
A)a hydrocarbon where carbon has only a single bond to two or more other carbons
B)a hydrocarbon that is completely dissolved in water
C)a hydrocarbon where carbon has multiple bonds to one or more other carbons
D)any molecule that is completely dissolved in a hydrocarbon solvent
E)none of the above
A)a hydrocarbon where carbon has only a single bond to two or more other carbons
B)a hydrocarbon that is completely dissolved in water
C)a hydrocarbon where carbon has multiple bonds to one or more other carbons
D)any molecule that is completely dissolved in a hydrocarbon solvent
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is a heteroatom?
A)O
B)C
C)H
D)A and B
E)all of the above
A)O
B)C
C)H
D)A and B
E)all of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the above sections of this molecule would be considered a saturated portion of the molecule?
A)a
B)b
C)c
D)a and b
E)all of the above
A)a
B)b
C)c
D)a and b
E)all of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
31
What property of carbon allows for the formation of so many different organic molecules?
A)Carbon forms three different isotopes which allows for the formation of many more molecules than other elements.
B)Carbon atoms are unique in their ability to form strong chemical bonds repeatedly with other carbon atoms, which permits the formation of countless possible structures.
C)Carbon atoms uniquely contain exactly the same number (six)of protons, neutrons and electrons in their atomic structure.
D)Carbon resides in the exact middle of the periodic table, from left to right, with exactly the same number of reactive elements on either side of it. It can therefore react with the maximum number of elements.
A)Carbon forms three different isotopes which allows for the formation of many more molecules than other elements.
B)Carbon atoms are unique in their ability to form strong chemical bonds repeatedly with other carbon atoms, which permits the formation of countless possible structures.
C)Carbon atoms uniquely contain exactly the same number (six)of protons, neutrons and electrons in their atomic structure.
D)Carbon resides in the exact middle of the periodic table, from left to right, with exactly the same number of reactive elements on either side of it. It can therefore react with the maximum number of elements.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following does not describe the number of other atoms a carbon atom can be bonded to?
A)only 2
B)only 3
C)only 4
D)only 5
A)only 2
B)only 3
C)only 4
D)only 5

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
33
Carbon-carbon single bonds can rotate while carbon-carbon double bonds cannot rotate. How many different structures are shown below. 
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
34
How many structural isomers are shown here? 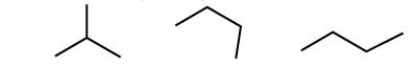
A)one
B)two
C)three
D)four

A)one
B)two
C)three
D)four

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is not an unsaturated molecule? 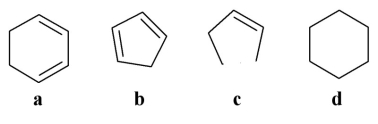
A)a
B)b
C)c
D)d
E)All of the above are unsaturated.

A)a
B)b
C)c
D)d
E)All of the above are unsaturated.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the above sections of this molecule would be considered to be the aromatic portion of the molecule?
A)a
B)b
C)c
D)a and b
E)all of the above
A)a
B)b
C)c
D)a and b
E)all of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
37
Which two of these four structures are of the same structural isomer? 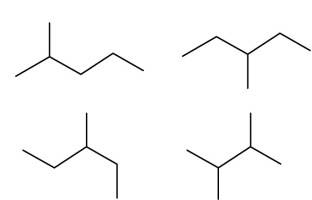
A)upper right and lower left
B)upper left and lower right
C)lower left and upper right
D)lower right and upper right

A)upper right and lower left
B)upper left and lower right
C)lower left and upper right
D)lower right and upper right

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
38
The temperatures in a fractionating tower at an oil refinery are important, but so are the pressures. Where might the pressure in a fractional distillation tower be greatest, at the bottom or at the top? Defend your answer.
A)The pressure is greatest at the bottom because the higher temperature means a greater number of vaporized molecules.
B)The pressure is greatest at the top because the higher temperature means a greater number of vaporized molecules.
C)The pressure is greatest at the bottom because the lower temperature means a greater number of vaporized molecules.
D)The pressure is greatest at the top because the lower temperature means a greater number of vaporized molecules.
A)The pressure is greatest at the bottom because the higher temperature means a greater number of vaporized molecules.
B)The pressure is greatest at the top because the higher temperature means a greater number of vaporized molecules.
C)The pressure is greatest at the bottom because the lower temperature means a greater number of vaporized molecules.
D)The pressure is greatest at the top because the lower temperature means a greater number of vaporized molecules.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is an aromatic molecule? 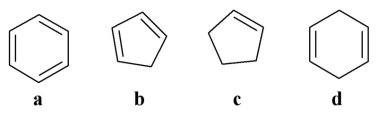
A)a
B)b
C)c
D)d
E)all of the above

A)a
B)b
C)c
D)d
E)all of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is a saturated molecule? 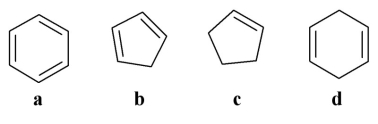
A)a
B)b
C)c
D)d
E)none of the above

A)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
41
What is the percent volume of water in 80-proof vodka?
A)80 percent
B)60 percent
C)40 percent
D)20 percent
A)80 percent
B)60 percent
C)40 percent
D)20 percent

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
42
If an alcoholic beverage is 20 percent alcohol by volume, what is its proof?
A)40 proof
B)20 proof
C)10 proof
D)Proof is a measure of flammability.
E)Proof is a measure of the age of a fermented beverage.
A)40 proof
B)20 proof
C)10 proof
D)Proof is a measure of flammability.
E)Proof is a measure of the age of a fermented beverage.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
43
How does ingested methanol lead to the damaging of a person's eyes?
A)Eye tissues are soluble in methanol.
B)The methanol is metabolized into formaldehyde, which is toxic to most living tissue.
C)Methanol is very volatile. When brought close to the eyes, its vapors can harm the eyes.
D)The methanol, once in the eyes, forms a crystalline precipitate that accumulates within the retina.
A)Eye tissues are soluble in methanol.
B)The methanol is metabolized into formaldehyde, which is toxic to most living tissue.
C)Methanol is very volatile. When brought close to the eyes, its vapors can harm the eyes.
D)The methanol, once in the eyes, forms a crystalline precipitate that accumulates within the retina.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
44
Correctly identify the following functional groups in this organic molecule-amide, ester, ketone, ether, alcohol, aldehyde, amine. 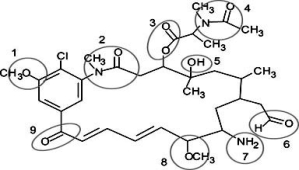
A)1 = ether, 3 = ester, 6 = aldehyde, 9 = alcohol
B)2 = amide, 4 = ester, 7 = amine, 8 = ether
C)1 = ester, 5 = alcohol, 8 = ether, 9 = ketone
D)2 = amide, 6 = aldehyde, 7 = amine, 8 = ether

A)1 = ether, 3 = ester, 6 = aldehyde, 9 = alcohol
B)2 = amide, 4 = ester, 7 = amine, 8 = ether
C)1 = ester, 5 = alcohol, 8 = ether, 9 = ketone
D)2 = amide, 6 = aldehyde, 7 = amine, 8 = ether

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
45
Why is 2,4,5-trifluorophenol much more acidic than phenol? 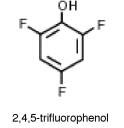
A)The fluorines adjacent to the OH group are large and shield the hydroxl group from making the solution basic.
B)The fluorines de-stabilize the benzene ring and thus give the structure more acidic characteristics.
C)The fluorines have high electronegativities and help to stabilize the negative charges, which makes it even easier for the hydrogen to be lost.
D)False! 2,4,5-trifluorophenol is actually not more acidic than phenol.

A)The fluorines adjacent to the OH group are large and shield the hydroxl group from making the solution basic.
B)The fluorines de-stabilize the benzene ring and thus give the structure more acidic characteristics.
C)The fluorines have high electronegativities and help to stabilize the negative charges, which makes it even easier for the hydrogen to be lost.
D)False! 2,4,5-trifluorophenol is actually not more acidic than phenol.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
46
Why is a phenol more acidic than a regular alcohol?
A)Phenols have the ability to spread the negative charge that forms.
B)Phenols have the ability to readily accept the positive charge of the proton.
C)The negative charge is very concentrated on the oxygen ring of the phenol.
D)The phenol has more negative charge and therefore is more stable.
E)Phenols are not more acidic than a regular alcohol.
A)Phenols have the ability to spread the negative charge that forms.
B)Phenols have the ability to readily accept the positive charge of the proton.
C)The negative charge is very concentrated on the oxygen ring of the phenol.
D)The phenol has more negative charge and therefore is more stable.
E)Phenols are not more acidic than a regular alcohol.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following compounds is likely to have the best water solubility?
A)CH₃CH₂OH
B)CH₃CH₂CH₂CH₂OH
C)CH₃CH₂CH₂CH₂CH₂CH₂OH
D)CH₃CH₂CH₂CH₂CH₂CH₂CH₂CH₂OH
A)CH₃CH₂OH
B)CH₃CH₂CH₂CH₂OH
C)CH₃CH₂CH₂CH₂CH₂CH₂OH
D)CH₃CH₂CH₂CH₂CH₂CH₂CH₂CH₂OH

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
48
One of the skin-irritating components of poison oak is tetrahydrourushiol: 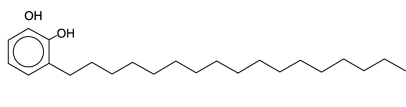 The long, nonpolar hydrocarbon tail embeds itself in a person's oily skin, where the molecule initiates an allergic response. Scratching the itch spreads tetrahydrourushiol molecules over a greater surface area, causing the zone of irritation to grow. Is this compound an alcohol or a phenol?
The long, nonpolar hydrocarbon tail embeds itself in a person's oily skin, where the molecule initiates an allergic response. Scratching the itch spreads tetrahydrourushiol molecules over a greater surface area, causing the zone of irritation to grow. Is this compound an alcohol or a phenol?
A)an alcohol because of the hydroxyl groups it contains
B)both a phenol and an alcohol because of the hydroxyl groups it contains
C)a phenol because of the hydroxyl groups attached to the benzene ring
D)neither an alcohol or phenol because it contains two hydroxyl groups instead of one
 The long, nonpolar hydrocarbon tail embeds itself in a person's oily skin, where the molecule initiates an allergic response. Scratching the itch spreads tetrahydrourushiol molecules over a greater surface area, causing the zone of irritation to grow. Is this compound an alcohol or a phenol?
The long, nonpolar hydrocarbon tail embeds itself in a person's oily skin, where the molecule initiates an allergic response. Scratching the itch spreads tetrahydrourushiol molecules over a greater surface area, causing the zone of irritation to grow. Is this compound an alcohol or a phenol?A)an alcohol because of the hydroxyl groups it contains
B)both a phenol and an alcohol because of the hydroxyl groups it contains
C)a phenol because of the hydroxyl groups attached to the benzene ring
D)neither an alcohol or phenol because it contains two hydroxyl groups instead of one

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
49
Why do ethers have lower boiling points than that of an alcohol of the same molecular weight?
A)Ethers cannot hydrogen bond to other ethers.
B)Ethers do not have hydrogens.
C)Ethers are more flammable and vaporize more easily.
D)Ethers have higher boiling points than alcohols.
E)all of the above
A)Ethers cannot hydrogen bond to other ethers.
B)Ethers do not have hydrogens.
C)Ethers are more flammable and vaporize more easily.
D)Ethers have higher boiling points than alcohols.
E)all of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
50
If champagne has a proof of 28, how many milliliters of alcohol are in 250 mL of champagne?
A)35
B)38
C)28
D)3)8
E)200
A)35
B)38
C)28
D)3)8
E)200

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
51
List the following compounds in order of least oxidized to most oxidized 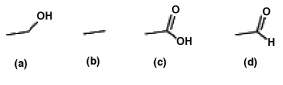
A)a < b < c < d
B)b < a < c < d
C)c < d < a < b
D)b < a < d < c

A)a < b < c < d
B)b < a < c < d
C)c < d < a < b
D)b < a < d < c

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
52
Formaldehyde is a toxic preservative with the following chemical formula: H₂C  O Which of the following compounds would best serve as a starting point for its production?
O Which of the following compounds would best serve as a starting point for its production?
A)H₃COH
B)H₂C CH₂
CH₂
C)H₂O
D)CH₃CH₂OH
E)none of the above
 O Which of the following compounds would best serve as a starting point for its production?
O Which of the following compounds would best serve as a starting point for its production?A)H₃COH
B)H₂C
 CH₂
CH₂C)H₂O
D)CH₃CH₂OH
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
53
Why might a high-formula-mass alcohol be insoluble in water?
A)A high formula mass alcohol would be too attracted to itself to be soluble in water.
B)The bulk of a high formula mass alcohol likely consists of nonpolar hydrocarbons.
C)Such an alcohol would likely be in a solid phase.
D)In order for two substance to be soluble in each other their molecules need to be of comparable mass.
A)A high formula mass alcohol would be too attracted to itself to be soluble in water.
B)The bulk of a high formula mass alcohol likely consists of nonpolar hydrocarbons.
C)Such an alcohol would likely be in a solid phase.
D)In order for two substance to be soluble in each other their molecules need to be of comparable mass.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
54
Which region (a-b-c)is a phenolic functional group?
A)a
B)b
C)c
D)b or c
E)b and c
A)a
B)b
C)c
D)b or c
E)b and c

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following compounds is an amine? 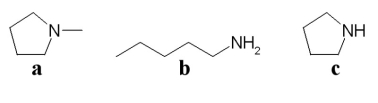
A)a
B)b
C)c
D)all of the above
E)only b and c

A)a
B)b
C)c
D)all of the above
E)only b and c

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
56
What is a functional group?
A)a set of atoms bonded together that give molecules containing them similar properties
B)a group that adds function
C)a group of hydrocarbons that can be used to make new materials
D)a set of molecules that are grouped together to form a functional material
E)none of the above
A)a set of atoms bonded together that give molecules containing them similar properties
B)a group that adds function
C)a group of hydrocarbons that can be used to make new materials
D)a set of molecules that are grouped together to form a functional material
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
57
Heteroatoms make a difference in the physical and chemical properties of an organic molecule because
A)they add extra mass to the hydrocarbon structure.
B)each heteroatom has its own characteristic chemistry.
C)they can enhance the polarity of the organic molecule.
D)all of the above
A)they add extra mass to the hydrocarbon structure.
B)each heteroatom has its own characteristic chemistry.
C)they can enhance the polarity of the organic molecule.
D)all of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following molecules contains a phenol? 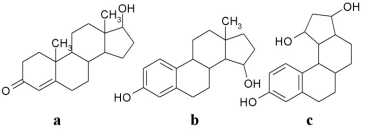
A)a
B)b
C)c
D)b and c
E)all of them

A)a
B)b
C)c
D)b and c
E)all of them

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
59
If an alcoholic beverage has a proof of 151, what is the percentage of alcohol in the beverage?
A)75.5 percent
B)151 percent
C)15.1 percent
D)Proof is a measure of flammability.
E)Proof is a measure of the age of a fermented beverage.
A)75.5 percent
B)151 percent
C)15.1 percent
D)Proof is a measure of flammability.
E)Proof is a measure of the age of a fermented beverage.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following ethers has the highest boiling point?
A)CH₃OCH₃
B)CH₃CH₂OCH₂CH₃
C)CH₃CH₂CH₂OCH₂CH₂CH₃
D)CH₃CH₂CH₂CH₂OCH₂CH₂CH₂CH₃
A)CH₃OCH₃
B)CH₃CH₂OCH₂CH₃
C)CH₃CH₂CH₂OCH₂CH₂CH₃
D)CH₃CH₂CH₂CH₂OCH₂CH₂CH₂CH₃

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
61
Suggest an explanation for why aspirin has a sour taste.
A)It is the acidic nature of aspirin that gives rise to its sour taste.
B)The sour flavor is added to help prevent overdosing.
C)Aspirin is made sour as a mandated child safety feature.
D)It is the basic nature of aspirin that gives rise to its sour taste.
A)It is the acidic nature of aspirin that gives rise to its sour taste.
B)The sour flavor is added to help prevent overdosing.
C)Aspirin is made sour as a mandated child safety feature.
D)It is the basic nature of aspirin that gives rise to its sour taste.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
62
The amino acid lysine is shown below. What functional group must be removed in order to produce cadaverine (1,5-pentanediamine) 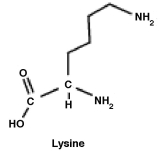
A)One amino group must be removed and replaced with a hydrogen.
B)The carboxyl group must be removed and replaced with a hydrogen.
C)The hydrogen (shown)must be removed and replaced with an amino (NH₂)group.
D)Lysine is 1, 5-pentanediamine. Nothing has to be removed.

A)One amino group must be removed and replaced with a hydrogen.
B)The carboxyl group must be removed and replaced with a hydrogen.
C)The hydrogen (shown)must be removed and replaced with an amino (NH₂)group.
D)Lysine is 1, 5-pentanediamine. Nothing has to be removed.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
63
Explain why caprylic acid, CH3(CH2)6COOH, dissolves in a 5 percent aqueous solution of sodium hydroxide but caprylaldehyde, CH3(CH2)6CHO, does not.
A)With two oxygens, the caprylic acid is about twice as polar the caprylaldehyde.
B)The caprylaldehyde is a gas at room temperature.
C)The caprylaldehyde behaves as a reducing agent, which neutralizes the sodium hydroxide.
D)The caprylic acid reacts to form the water soluble salt.
A)With two oxygens, the caprylic acid is about twice as polar the caprylaldehyde.
B)The caprylaldehyde is a gas at room temperature.
C)The caprylaldehyde behaves as a reducing agent, which neutralizes the sodium hydroxide.
D)The caprylic acid reacts to form the water soluble salt.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the above molecules is a ketone?
A)a
B)b
C)c
D)d
E)none of the above
A)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
65
An amino acid is an organic molecule that contains both an amine group and a carboxyl group. At an acidic pH, which structure is most likely? 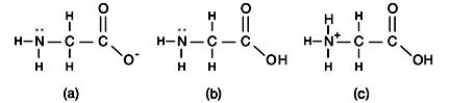
A)Structure (a)
B)Structure (b)
C)Structure (c)
D)All three structures are equally possible.

A)Structure (a)
B)Structure (b)
C)Structure (c)
D)All three structures are equally possible.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
66
The solvent diethyl ether can be mixed with water but only by shaking the two liquids together. After the shaking is stopped, the liquids separate into two layers, much like oil and vinegar. The free-base form of the alkaloid caffeine is readily soluble in diethyl ether but not in water. Suggest what might happen to the caffeine of a caffeinated beverage if the beverage was first made alkaline with sodium hydroxide and then shaken with some diethyl ether.
A)The water layer would turn a pink color indicating an alkaline pH.
B)The caffeine would transform into the free base and transfer into the diethyl ether.
C)The caffeine would transform into the free acid and transfer into the diethyl ether.
D)The diethyl ether and water would mix into one layer.
A)The water layer would turn a pink color indicating an alkaline pH.
B)The caffeine would transform into the free base and transfer into the diethyl ether.
C)The caffeine would transform into the free acid and transfer into the diethyl ether.
D)The diethyl ether and water would mix into one layer.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the above molecules is a phenol?
A)a
B)b
C)c
D)d
E)a and d
A)a
B)b
C)c
D)d
E)a and d

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
68
The phosphoric acid salt of caffeine has the structure 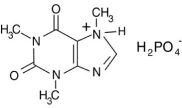 This molecule behaves as an acid in that it can donate a hydrogen ion, created from the hydrogen atom bonded to the positively charged nitrogen atom. What salt is formed when 1 mole of this salt reacts with 1 mole of sodium hydroxide, NaOH, a strong base?
This molecule behaves as an acid in that it can donate a hydrogen ion, created from the hydrogen atom bonded to the positively charged nitrogen atom. What salt is formed when 1 mole of this salt reacts with 1 mole of sodium hydroxide, NaOH, a strong base?
A)Salts can't react to form salts, rather they only arise from the reaction of an acid and a base.
B)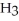
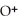
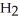 P
P 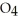

C)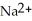 HP
HP 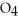
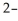
D)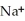
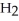 P
P 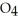

 This molecule behaves as an acid in that it can donate a hydrogen ion, created from the hydrogen atom bonded to the positively charged nitrogen atom. What salt is formed when 1 mole of this salt reacts with 1 mole of sodium hydroxide, NaOH, a strong base?
This molecule behaves as an acid in that it can donate a hydrogen ion, created from the hydrogen atom bonded to the positively charged nitrogen atom. What salt is formed when 1 mole of this salt reacts with 1 mole of sodium hydroxide, NaOH, a strong base?A)Salts can't react to form salts, rather they only arise from the reaction of an acid and a base.
B)
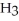
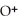
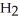 P
P 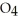

C)
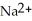 HP
HP 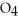
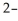
D)
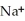
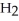 P
P 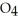


Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
69
If you saw the label "phenylephrine HCl" on a decongestant, would you worry that consuming it would expose you to the strong acid hydrochloric acid, HCl? 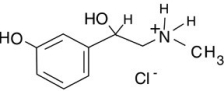
A)No, because it is balanced by the phenylephrine.
B)No, because the drug is in the solid phase.
C)No, because this is a salt made using hydrogen chloride, but it is in no way hydrogen chloride.
D)Yes, because of the hydrochloric acid it contains.

A)No, because it is balanced by the phenylephrine.
B)No, because the drug is in the solid phase.
C)No, because this is a salt made using hydrogen chloride, but it is in no way hydrogen chloride.
D)Yes, because of the hydrochloric acid it contains.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the above molecules is an aldehyde?
A)a
B)b
C)c
D)d
E)none of the above
A)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
71
An amine can often form R-NH₃+ (where R- can stand for anything)by reacting with water. What is happening?
A)The amine is accepting a proton from the water molecule, forming OH-.
B)Amines are basic.
C)Amines are acidic.
D)Amines can form H₃O+ in water by donating a proton from the polar portion of the molecule.
E)none of the above
A)The amine is accepting a proton from the water molecule, forming OH-.
B)Amines are basic.
C)Amines are acidic.
D)Amines can form H₃O+ in water by donating a proton from the polar portion of the molecule.
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
72
In water, does the molecule lysergic acid diethylamide act as an acid, a base, neither or both? 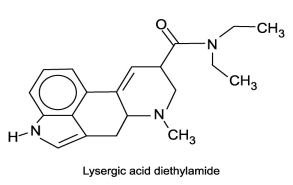
A)acid
B)base
C)neither an acid or a base
D)both as an acid and as a base

A)acid
B)base
C)neither an acid or a base
D)both as an acid and as a base

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
73
Alkaloid salts are not very soluble in the organic solvent diethyl ether. What might happen to the free-base form of caffeine dissolved in diethyl ether if gaseous hydrogen chloride, HCl, were bubbled into the solution? 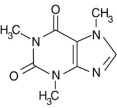
A)A second layer of water would form.
B)Nothing, and the HCl gas would merely bubble out of solution.
C)The diethyl ether insoluble caffeine salt would form as a white precipitate.
D)The acid/base reaction would release heat, which would cause the diethyl ether to start evaporating.

A)A second layer of water would form.
B)Nothing, and the HCl gas would merely bubble out of solution.
C)The diethyl ether insoluble caffeine salt would form as a white precipitate.
D)The acid/base reaction would release heat, which would cause the diethyl ether to start evaporating.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following commonly acts as a base in chemical reactions?
A)carboxylic acid
B)alcohol
C)ketone
D)amine
E)hydrocarbon
A)carboxylic acid
B)alcohol
C)ketone
D)amine
E)hydrocarbon

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the above molecules is a carboxylic acid?
A)a
B)b
C)c
D)d
E)none of the above
A)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the above molecules would most likely have a strong, distinct smell?
A)a
B)b
C)c
D)d
E)a or d
A)a
B)b
C)c
D)d
E)a or d

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the above molecules contains a carbonyl group?
A)a
B)all but a
C)c
D)all but b
E)all but d
A)a
B)all but a
C)c
D)all but b
E)all but d

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
78
A common inactive ingredient in products such as sunscreen lotions and shampoo is triethyl amine, also known as TEA. Which of the following is the chemical structure for this compound? 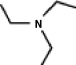
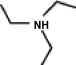
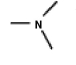
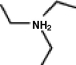 (a)(b)(c)(d)
(a)(b)(c)(d)
A)a
B)b
C)c
D)d



 (a)(b)(c)(d)
(a)(b)(c)(d)A)a
B)b
C)c
D)d

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
79
How many structural isomers are there for a compound having the molecular formula C₃H9N?
A)one
B)two
C)three
D)four
A)one
B)two
C)three
D)four

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the above molecules would be acidic?
A)a
B)b
C)c
D)d
E)a or d
A)a
B)b
C)c
D)d
E)a or d

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck


