Deck 13: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/252
Play
Full screen (f)
Deck 13: Chemical Reactions
1
Which equations are balanced? a)Mg (s)+ 2HCl (aq)→ MgCl2 (aq)+ H₂ (g)
B)3Al (s)+ 3 Br2 (l)→ Al2Br3 (s)
C)2HgO (s)→ 2 Hg (l)+ O₂ (g)
A)Only equation "c" is balanced.
B)Equations "a" and "c" are balanced.
C)Equations "b" and "c" are balanced.
D)All of them are balanced.
B)3Al (s)+ 3 Br2 (l)→ Al2Br3 (s)
C)2HgO (s)→ 2 Hg (l)+ O₂ (g)
A)Only equation "c" is balanced.
B)Equations "a" and "c" are balanced.
C)Equations "b" and "c" are balanced.
D)All of them are balanced.
B
2
What is a chemical equation?
A)It is a shorthand notation for illustrating a chemical reaction.
B)It is the sum of the masses of the products and reactants.
C)It is the chemical combination of equal numbers of reactants and products.
D)It is a picture of the atoms undergoing a chemical equalization.
E)It is any type of reaction that takes place at the equator.
A)It is a shorthand notation for illustrating a chemical reaction.
B)It is the sum of the masses of the products and reactants.
C)It is the chemical combination of equal numbers of reactants and products.
D)It is a picture of the atoms undergoing a chemical equalization.
E)It is any type of reaction that takes place at the equator.
A
3
Balance the following chemical equation. ____ N2 + ____ H₂ → ____ NH₃
A)1, 3, 2
B)1, 2, 3
C)3, 2, 1
D)2, 6, 4
E)1/2, 3/2, 1
A)1, 3, 2
B)1, 2, 3
C)3, 2, 1
D)2, 6, 4
E)1/2, 3/2, 1
A
4
Which of the following is a correctly balanced equation?
A)P4 + 6 H₂ → 4 PH₃
B)1 P4 + 6 H₂ → 4 PH₃
C)0 P4 + 6 H₂ → 4 PH₃
D)2 P4 + 12 H₂ → 8 PH₃
E)P4 + 3 H₂ → PH₃
A)P4 + 6 H₂ → 4 PH₃
B)1 P4 + 6 H₂ → 4 PH₃
C)0 P4 + 6 H₂ → 4 PH₃
D)2 P4 + 12 H₂ → 8 PH₃
E)P4 + 3 H₂ → PH₃

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
5
Balance these equations. ____ H₂ (g)+ ____ N2 (g)→ ____ NH₃ (g)
A)2, 2, 3
B)2, 2, 5
C)3, 3, 2
D)3, 1, 2
A)2, 2, 3
B)2, 2, 5
C)3, 3, 2
D)3, 1, 2

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
6
Given the following generic chemical reaction, which is the reactant? X → Y
A)Y is the reactant.
B)X is the reactant.
C)→ is the reactant.
D)Both X and Y are the products.
E)Both X and Y are the reactants.
A)Y is the reactant.
B)X is the reactant.
C)→ is the reactant.
D)Both X and Y are the products.
E)Both X and Y are the reactants.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
7
What is a chemical reaction?
A)when one or more new compounds are formed by rearranging atoms
B)when a new element is formed by rearranging nucleons
C)when two solids mix together to form a heterogeneous mixture
D)when two liquids mix to form a homogeneous mixture
E)when a liquid undergoes a phase change and produces a solid
A)when one or more new compounds are formed by rearranging atoms
B)when a new element is formed by rearranging nucleons
C)when two solids mix together to form a heterogeneous mixture
D)when two liquids mix to form a homogeneous mixture
E)when a liquid undergoes a phase change and produces a solid

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
8
Which equation best describes the reaction represented in the illustration above?
A)2 AB2 + 2 DCB3 + B2 → 2 DBA4 + 2 CA2
B)2 AB2 + 2 CDA3 + B2 → 2 C2A4 + 2 DBA
C)2 AB2 + 2 CDA3 + A2 → 2 DBA4 + 2 CA2
D)2 BA2 + 2 CDA3 + A2 → 2 DBA4 + 2 CA2
A)2 AB2 + 2 DCB3 + B2 → 2 DBA4 + 2 CA2
B)2 AB2 + 2 CDA3 + B2 → 2 C2A4 + 2 DBA
C)2 AB2 + 2 CDA3 + A2 → 2 DBA4 + 2 CA2
D)2 BA2 + 2 CDA3 + A2 → 2 DBA4 + 2 CA2

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
9
Balance the following equation. ____ NO → ____ N2O + _____ NO₂
A)3, 1, 1
B)3, 0, 0
C)4, 4, 8
D)1, 2, 4
E)6, 2, 1
A)3, 1, 1
B)3, 0, 0
C)4, 4, 8
D)1, 2, 4
E)6, 2, 1

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
10
How many diatomic molecules are represented in the illustration above?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
11
Given the following generic chemical reaction, which is the product? X → Y
A)Y is the product.
B)X is the product.
C)→ is the product.
D)Both X and Y are the products.
E)Both X and Y are the reactants.
A)Y is the product.
B)X is the product.
C)→ is the product.
D)Both X and Y are the products.
E)Both X and Y are the reactants.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
12
What coefficients balance the following equation? ____ P4 (s)+ ____ H₂ (g)→ ____ PH₃ (g)
A)4, 2, 3
B)1, 6, 4
C)1, 4, 4
D)2, 10, 8
A)4, 2, 3
B)1, 6, 4
C)1, 4, 4
D)2, 10, 8

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
13
Steel wool wetted with vinegar is sealed within a balloon inflated with air. After several hours, what happens to the volume of the balloon?
A)The balloon inflates.
B)The balloon deflates.
C)The balloon dissolves.
D)Nothing because the vinegar is acting on the steel wool, not upon the balloon.
A)The balloon inflates.
B)The balloon deflates.
C)The balloon dissolves.
D)Nothing because the vinegar is acting on the steel wool, not upon the balloon.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
14
What coefficient is needed in front of the O₂ molecule to balance the following equation? 2 C4H10 (g)+ _____ O₂ (g)→ 8 CO₂ (g)+ 10 H₂O (l)
A)8
B)13
C)5
D)1
A)8
B)13
C)5
D)1

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
15
For the following balanced reaction, which of the following is a solid? 2 Na(l)+ Cl2(g)→ 2 NaCl(s)
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
16
What is wrong with the following depiction of a chemical reaction? 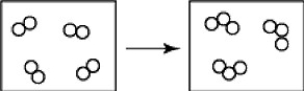
A)These boxes contain only molecules but no atoms.
B)One box contains more molecules than the other.
C)One box contains more atoms than the other.
D)all of the above

A)These boxes contain only molecules but no atoms.
B)One box contains more molecules than the other.
C)One box contains more atoms than the other.
D)all of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
17
For the following balanced equation, which has the highest coefficient? 4 H₂ + 2 C → 2 CH₄
A)H₂
B)C
C)CH₄
D)H₄
E)none of the above
A)H₂
B)C
C)CH₄
D)H₄
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
18
Steel wool wetted with vinegar is stuffed into a narrow mouth round glass bottle. A rubber balloon is then sealed over the mouth of the bottle. After several hours, the balloon inflates into the bottle in an inverted manner. What happened?
A)Vinegar fumes are diamagnetic and as they accumulate above the liquid the steel wool is attracted thus inflating the balloon into the mouth of the bottle in an inverted manner.
B)The caustic vinegar fumes get past the steel wool and deteriorate the balloon, which begins to sag into the bottle and inflate it in an inverted manner.
C)The vinegar reacts with the steel wool by absorbing oxygen within the bottle thus decreasing the pressure. The greater outside pressure causes the balloon to inflate in an inverted manner.
D)False! The balloon inflates above the mouth of the bottle because the reaction between the vinegar and steel wool produces a gas which is forced upward because of increased pressure inside the sealed bottle.
A)Vinegar fumes are diamagnetic and as they accumulate above the liquid the steel wool is attracted thus inflating the balloon into the mouth of the bottle in an inverted manner.
B)The caustic vinegar fumes get past the steel wool and deteriorate the balloon, which begins to sag into the bottle and inflate it in an inverted manner.
C)The vinegar reacts with the steel wool by absorbing oxygen within the bottle thus decreasing the pressure. The greater outside pressure causes the balloon to inflate in an inverted manner.
D)False! The balloon inflates above the mouth of the bottle because the reaction between the vinegar and steel wool produces a gas which is forced upward because of increased pressure inside the sealed bottle.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
19
A friend argues that if mass were really conserved he would never need to refill his gas tank. What explanation do you offer your friend?
A)The atoms (mass)of gasoline are converted into energy by the engine according to E=m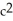 .
.
B)The Law of Conservation of Mass does not apply to reactions involving combustion or explosion of matter.
C)The atoms (mass)of gasoline are converted into exhaust fumes.
D)The oil companies make gasoline in a way that it gets used up so that we are always required to replenish it.
A)The atoms (mass)of gasoline are converted into energy by the engine according to E=m
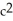 .
.B)The Law of Conservation of Mass does not apply to reactions involving combustion or explosion of matter.
C)The atoms (mass)of gasoline are converted into exhaust fumes.
D)The oil companies make gasoline in a way that it gets used up so that we are always required to replenish it.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
20
For the following balanced reaction, which of the following is a gas? 2 Na(l)+ Cl2(g)→ 2 NaCl(s)
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
21
Bond energies increase in going from C-N (lowest)to C-O to C-F (highest). Explain this trend based upon the atomic sizes of these atoms as deduced from their positions in the periodic table.
A)In going from nitrogen to oxygen to fluorine the atoms get larger. This means a greater nuclear charge, which translates into stronger chemical bonds.
B)In going from nitrogen to oxygen to fluorine the atoms get smaller. This means a greater nuclear charge, which translates into stronger chemical bonds.
C)In going from nitrogen to oxygen to fluorine the atoms get larger. This means that the bonding atoms are farther apart, which translates into a greater bond energy.
D)In going from nitrogen to oxygen to fluorine the atoms get smaller. This means that the bonding atoms are closer together, which translates into a greater bond energy.
A)In going from nitrogen to oxygen to fluorine the atoms get larger. This means a greater nuclear charge, which translates into stronger chemical bonds.
B)In going from nitrogen to oxygen to fluorine the atoms get smaller. This means a greater nuclear charge, which translates into stronger chemical bonds.
C)In going from nitrogen to oxygen to fluorine the atoms get larger. This means that the bonding atoms are farther apart, which translates into a greater bond energy.
D)In going from nitrogen to oxygen to fluorine the atoms get smaller. This means that the bonding atoms are closer together, which translates into a greater bond energy.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
22
How many moles of H₂ bonds are broken if you react nitrogen with hydrogen according to the following reaction? N2 + 3H₂ → 2 NH₃
A)6
B)2
C)3
D)1
E)8
A)6
B)2
C)3
D)1
E)8

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
23
Given that the above energy profiles have the same scale, which of the reactions is the most exothermic?
A)a
B)b
C)c
D)d
E)none of the above
A)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
24
How much energy, in kilojoules, is released or absorbed from the reaction of one mole of nitrogen, N2, with three moles of molecular hydrogen, H₂, to form two moles of ammonia, NH₃? H-N (bond energy: 389 kJ/mol)
H-H (bond energy: 436 kJ/mol)
N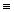 N (bond energy: 946 kJ/mol)
N (bond energy: 946 kJ/mol)
N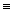 N + H-H + H-H → NH₃ + NH₃
N + H-H + H-H → NH₃ + NH₃
A)+899 kJ/mol absorbed
B)-993 kJ/mol released
C)+80 kJ/mol absorbed
D)-80 kj/mol released
H-H (bond energy: 436 kJ/mol)
N
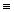 N (bond energy: 946 kJ/mol)
N (bond energy: 946 kJ/mol)N
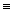 N + H-H + H-H → NH₃ + NH₃
N + H-H + H-H → NH₃ + NH₃A)+899 kJ/mol absorbed
B)-993 kJ/mol released
C)+80 kJ/mol absorbed
D)-80 kj/mol released

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
25
Which is higher in an endothermic reaction: the potential energy of the reactants or the potential energy of the products?
A)The potential energy of the products is higher than the potential energy of the reactants.
B)The potential energy of the reactants is higher than the potential energy of the products.
C)The potential energy of the reactants is the same as the potential energy of the products.
D)In the early stages of the reaction the potential energy of the reactants is higher. In the later stages, the potential energy of the products is higher.
A)The potential energy of the products is higher than the potential energy of the reactants.
B)The potential energy of the reactants is higher than the potential energy of the products.
C)The potential energy of the reactants is the same as the potential energy of the products.
D)In the early stages of the reaction the potential energy of the reactants is higher. In the later stages, the potential energy of the products is higher.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
26
Are the chemical reactions that take place in a disposable battery exothermic or endothermic? Is the reaction going on in a rechargeable battery while it is recharging exothermic or endothermic?
A)An operating disposable battery is driven by endothermic reaction, while a recharging rechargeable battery is also driven by endothermic reactions.
B)An operating disposable battery is driven by exothermic reaction, while a recharging rechargeable battery is driven by endothermic reactions.
C)An operating disposable battery is driven by endothermic reaction, while a recharging rechargeable battery is driven by exothermic reactions.
D)An operating disposable battery is driven by exothermic reaction, while a recharging rechargeable battery is also driven by exothermic reactions.
A)An operating disposable battery is driven by endothermic reaction, while a recharging rechargeable battery is also driven by endothermic reactions.
B)An operating disposable battery is driven by exothermic reaction, while a recharging rechargeable battery is driven by endothermic reactions.
C)An operating disposable battery is driven by endothermic reaction, while a recharging rechargeable battery is driven by exothermic reactions.
D)An operating disposable battery is driven by exothermic reaction, while a recharging rechargeable battery is also driven by exothermic reactions.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
27
Use the bond energies below to determine whether the following reaction is exothermic or endothermic: H₂ + Cl2 → 2 HCL
H-H (bond energy: 436 kJ/mol)
Cl-Cl (bond energy: 243 kJ/mol)
H-Cl (bond energy: 431 kJ/mol)
A)exothermic with more than 50 kJ of energy released
B)endothermic with more than 50 kJ of energy absorbed
C)exothermic with less than 50 kJ of energy released
D)endothermic with less than 50 kJ of energy absorbed
H-H (bond energy: 436 kJ/mol)
Cl-Cl (bond energy: 243 kJ/mol)
H-Cl (bond energy: 431 kJ/mol)
A)exothermic with more than 50 kJ of energy released
B)endothermic with more than 50 kJ of energy absorbed
C)exothermic with less than 50 kJ of energy released
D)endothermic with less than 50 kJ of energy absorbed

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following reaction energies is the least exothermic (but still an exothermic reaction)?
A)540 kJ/mole
B)-540 kJ/mole
C)125 kJ/mole
D)-125 kJ/mole
E)not enough information given
A)540 kJ/mole
B)-540 kJ/mole
C)125 kJ/mole
D)-125 kJ/mole
E)not enough information given

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
29
Given the following energy profiles, which of the following reactions is endothermic? 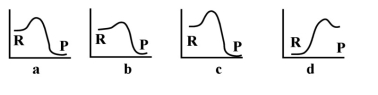 R= reactants P= products
R= reactants P= products
A)a
B)b
C)c
D)d
E)none of the above
 R= reactants P= products
R= reactants P= productsA)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
30
Given that the above energy profiles have the same scale, which of the reactions would require the most energy?
A)a
B)b
C)c
D)d
E)none of the above
A)a
B)b
C)c
D)d
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
31
What is a reaction rate?
A)It is the speed at which reactants are consumed or product is formed.
B)It is the balanced chemical formula that relates the number of product molecules to reactant molecules.
C)It is the ratio of the masses of products and reactants.
D)It is the ratio of the molecular masses of the elements in a given compound.
E)none of the above
A)It is the speed at which reactants are consumed or product is formed.
B)It is the balanced chemical formula that relates the number of product molecules to reactant molecules.
C)It is the ratio of the masses of products and reactants.
D)It is the ratio of the molecular masses of the elements in a given compound.
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
32
How many bonds between nitrogen and hydrogen are formed if you react nitrogen with hydrogen according to the following reaction? N2 + 3H₂ → 2 NH₃
A)6
B)2
C)3
D)1
E)8
A)6
B)2
C)3
D)1
E)8

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
33
What is an endothermic reaction?
A)It is a reaction that requires heat as a reactant.
B)It is a reaction where the products have more energy than the reactants.
C)It is a reaction where there is a net adsorption of energy from a reaction.
D)all of the above
E)none of the above
A)It is a reaction that requires heat as a reactant.
B)It is a reaction where the products have more energy than the reactants.
C)It is a reaction where there is a net adsorption of energy from a reaction.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
34
The reactants shown schematically below represent iron oxide, 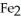
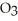 and carbon monoxide, CO. Which of the following is the correct full balanced chemical equation for what is depicted?
and carbon monoxide, CO. Which of the following is the correct full balanced chemical equation for what is depicted? 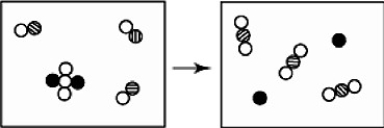
A)Fe₂O₃ + 3 CO → 2 Fe + 3 CO₂
B)Fe₂O₃ + 3 CO → 3 FeO + 2 C
C)Fe₂O₃ + 3 CO → 3 FeO₂ + 2C
D)Fe₂O₃ + 3 CO → 2 Fe + 3 C2O
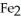
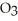 and carbon monoxide, CO. Which of the following is the correct full balanced chemical equation for what is depicted?
and carbon monoxide, CO. Which of the following is the correct full balanced chemical equation for what is depicted? 
A)Fe₂O₃ + 3 CO → 2 Fe + 3 CO₂
B)Fe₂O₃ + 3 CO → 3 FeO + 2 C
C)Fe₂O₃ + 3 CO → 3 FeO₂ + 2C
D)Fe₂O₃ + 3 CO → 2 Fe + 3 C2O

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
35
What is an exothermic reaction?
A)It is a reaction that requires heat as a reactant.
B)It is a reaction where the products have more energy than the reactants.
C)It is a reaction where there is a net adsorption of energy from a reaction.
D)all of the above
E)none of the above
A)It is a reaction that requires heat as a reactant.
B)It is a reaction where the products have more energy than the reactants.
C)It is a reaction where there is a net adsorption of energy from a reaction.
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
36
Given that the bond energy of N2 is 946 kJ/mole, the bond energy of O₂ is 498 kJ/mole and the NO bond energy is 631 kJ/mole, how much energy is required to react 1 mole of nitrogen molecules according to the following reaction? N2 + O₂ → 2 NO
A)182 kJ
B)-182 kJ
C)813 kJ
D)-813 kJ
E)2075 kJ
A)182 kJ
B)-182 kJ
C)813 kJ
D)-813 kJ
E)2075 kJ

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following reaction energies is the most endothermic?
A)540 kJ/mole
B)-540 kJ/mole
C)125 kj/mole
D)-125 kJ/mole
E)not enough information given
A)540 kJ/mole
B)-540 kJ/mole
C)125 kj/mole
D)-125 kJ/mole
E)not enough information given

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
38
Why might increasing the temperature alter the rate of a chemical reaction?
A)The molecules will have a higher kinetic energy and bump into one another harder.
B)The molecules are less reactive at higher temperatures.
C)The molecules will more likely combine with other atoms at high temperature to save space.
D)The density decreases as a function of temperature and this leads to an increase in volume which drops the rate of reaction.
E)none of the above
A)The molecules will have a higher kinetic energy and bump into one another harder.
B)The molecules are less reactive at higher temperatures.
C)The molecules will more likely combine with other atoms at high temperature to save space.
D)The density decreases as a function of temperature and this leads to an increase in volume which drops the rate of reaction.
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
39
If it takes energy to break bonds and you gain energy in the formation of bonds, how can some reactions be exothermic while others are endothermic?
A)It is the total amount of energy that matters. Sometimes some bonds are stronger than others and so you gain or lose energy when you form them.
B)It is the total number of bonds that matters. Sometimes you create more bonds than you break and since all bonds have same amount of energy you gain or lose energy depending on the number of bonds.
C)Some reactants have more energetic bonds than others and they will always release energy.
D)Some products have more energy than others and they always require energy to be formed.
E)none of the above
A)It is the total amount of energy that matters. Sometimes some bonds are stronger than others and so you gain or lose energy when you form them.
B)It is the total number of bonds that matters. Sometimes you create more bonds than you break and since all bonds have same amount of energy you gain or lose energy depending on the number of bonds.
C)Some reactants have more energetic bonds than others and they will always release energy.
D)Some products have more energy than others and they always require energy to be formed.
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
40
Why might increasing the concentration of a set of reactants increase the rate of reaction?
A)You have increased the chances that any two reactant molecules will collide and react.
B)You have increased the ratio of reactants to products.
C)The concentration of reactants is unrelated to the rate of reaction.
D)The rate of reaction depends only on the mass of the atoms and therefore increases as you increase the mass of the reactants.
E)none of the above
A)You have increased the chances that any two reactant molecules will collide and react.
B)You have increased the ratio of reactants to products.
C)The concentration of reactants is unrelated to the rate of reaction.
D)The rate of reaction depends only on the mass of the atoms and therefore increases as you increase the mass of the reactants.
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
41
Is the synthesis of ozone, O₃, from oxygen, O₂, an example of an exothermic or endothermic reaction?
A)exothermic because ultraviolet light is emitted during its formation
B)endothermic because ultraviolet light is emitted during its formation
C)exothermic because ultraviolet light is absorbed during its formation
D)endothermic because ultraviolet light is absorbed during its formation
A)exothermic because ultraviolet light is emitted during its formation
B)endothermic because ultraviolet light is emitted during its formation
C)exothermic because ultraviolet light is absorbed during its formation
D)endothermic because ultraviolet light is absorbed during its formation

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following statements about ozone is true?
A)Ozone in the stratosphere is beneficial.
B)Ozone in the troposphere is a pollutant.
C)Ozone is generated from photochemical reactions of hydrocarbons.
D)none of the above
E)both a and b
A)Ozone in the stratosphere is beneficial.
B)Ozone in the troposphere is a pollutant.
C)Ozone is generated from photochemical reactions of hydrocarbons.
D)none of the above
E)both a and b

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
43
What is the activation energy?
A)the minimum amount of energy to break the bonds in reactants
B)the amount of energy required to activate a phase change
C)the energy difference between the reactants and the products
D)the amount of energy required to separate reactants from the products
E)the hill
A)the minimum amount of energy to break the bonds in reactants
B)the amount of energy required to activate a phase change
C)the energy difference between the reactants and the products
D)the amount of energy required to separate reactants from the products
E)the hill

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
44
Why does a glowing splint of wood burn only slowly in air, but rapidly in a burst of flames when placed in pure oxygen?
A)There is a greater number of collisions between the wood and oxygen molecules.
B)Oxygen is a flammable gas.
C)Pure oxygen is able to absorb carbon dioxide at a faster rate.
D)A glowing wood splint is actually extinguished within pure oxygen because there's no room for the smoke to expand.
A)There is a greater number of collisions between the wood and oxygen molecules.
B)Oxygen is a flammable gas.
C)Pure oxygen is able to absorb carbon dioxide at a faster rate.
D)A glowing wood splint is actually extinguished within pure oxygen because there's no room for the smoke to expand.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
45
An Alka-Seltzer antacid tablet bubbles vigorously when placed in water but only slowly when placed in an alcoholic beverage of the same temperature containing a 50:50 mix of alcohol and water. Propose a probable explanation involving the relationship between the speed of a reaction and molecular collisions.
A)The alcohol absorbs the carbon dioxide bubbles before they escape the liquid phase.
B)Alcohol molecules are more massive than water molecules, hence they move slower and their collisions are not as forceful.
C)The tablet reacts with water but not the alcohol.
D)In a 50 : 50 mix there are fewer water molecules for the antacid molecules to collide with.
A)The alcohol absorbs the carbon dioxide bubbles before they escape the liquid phase.
B)Alcohol molecules are more massive than water molecules, hence they move slower and their collisions are not as forceful.
C)The tablet reacts with water but not the alcohol.
D)In a 50 : 50 mix there are fewer water molecules for the antacid molecules to collide with.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
46
Why is heat often added to chemical reactions performed in the laboratory?
A)to allow a greater number of reactants to pass over the activation energy
B)to increase the rate at which reactant collide
C)to compensate for the natural tendency of energy to disperse
D)all of the above
A)to allow a greater number of reactants to pass over the activation energy
B)to increase the rate at which reactant collide
C)to compensate for the natural tendency of energy to disperse
D)all of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
47
The ________ is what needs to be overcome in a reaction so that it can proceed to the products.
A)activation energy
B)catalyst
C)entropy
D)thermodynamics
E)bond energy
A)activation energy
B)catalyst
C)entropy
D)thermodynamics
E)bond energy

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
48
A refrigerator delays the spoilage of food by
A)killing microorganisms.
B)slowing down the rate of chemical reactions within microorganisms.
C)expanding the water found within microorganisms.
D)diminishing the supply of oxygen to microorganisms.
A)killing microorganisms.
B)slowing down the rate of chemical reactions within microorganisms.
C)expanding the water found within microorganisms.
D)diminishing the supply of oxygen to microorganisms.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
49
If the following graphs are for the same reaction, which one is most likely the one with an added catalyst? 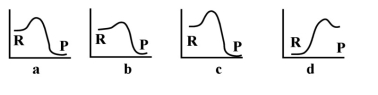
A)a
B)b
C)c
D)d
E)All have had catalyst added.

A)a
B)b
C)c
D)d
E)All have had catalyst added.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements accurately describes the action of ozone?
A)It absorbs UV radiation and undergoes fragmentation.
B)It reflects UV radiation back into space.
C)It reflects heat back into space.
D)It emits UV radiation when excited by sunlight.
E)none of the above
A)It absorbs UV radiation and undergoes fragmentation.
B)It reflects UV radiation back into space.
C)It reflects heat back into space.
D)It emits UV radiation when excited by sunlight.
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements about catalysts is NOT true?
A)A catalyst alters the rate of a chemical reaction.
B)A catalyst can be consumed in a reaction as long as it is regenerated.
C)A catalyst can be used to speed up slow reactions.
D)A catalyst does not change the energy of the reactants or the products.
E)All of the above are true.
A)A catalyst alters the rate of a chemical reaction.
B)A catalyst can be consumed in a reaction as long as it is regenerated.
C)A catalyst can be used to speed up slow reactions.
D)A catalyst does not change the energy of the reactants or the products.
E)All of the above are true.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
52
For the above energy profiles, which reaction has the highest activation energy?
A)a
B)b
C)c
D)d
E)All have the same activation energy.
A)a
B)b
C)c
D)d
E)All have the same activation energy.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
53
Some reactions are more sluggish than others. To speed up these reactions and save energy a(n)________ is sometimes added.
A)catalyst
B)activator
C)heat source
D)exotherm
E)reaction profile
A)catalyst
B)activator
C)heat source
D)exotherm
E)reaction profile

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
54
What can you deduce about the activation energy of a reaction that takes billions of years to go to completion? How about a reaction that takes only fractions of a second?
A)The activation energy of both these reactions must be very low.
B)The activation energy of both these reactions must be very high.
C)The slow reaction must have a high activation energy while the fast reaction must have a low activation energy.
D)The slow reaction must have a low activation energy while the fast reaction must have a high activation energy.
A)The activation energy of both these reactions must be very low.
B)The activation energy of both these reactions must be very high.
C)The slow reaction must have a high activation energy while the fast reaction must have a low activation energy.
D)The slow reaction must have a low activation energy while the fast reaction must have a high activation energy.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
55
What is the environmentally unfriendly component of chlorinated fluorocarbons that ultimately damages ozone?
A)chlorine atoms
B)fluorine atoms
C)carbon atoms
D)molecular fragments of carbon and fluorine
E)all of the above
A)chlorine atoms
B)fluorine atoms
C)carbon atoms
D)molecular fragments of carbon and fluorine
E)all of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
56
How does a catalyst increase the rate of a reaction?
A)It lowers the activation energy.
B)It is neither created nor consumed in a reaction.
C)It has nothing to do with the rate of reaction.
D)It increases the energy difference between the reactants and products.
E)It raises the activation energy of the reactants, which makes the reaction proceed faster.
A)It lowers the activation energy.
B)It is neither created nor consumed in a reaction.
C)It has nothing to do with the rate of reaction.
D)It increases the energy difference between the reactants and products.
E)It raises the activation energy of the reactants, which makes the reaction proceed faster.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
57
The yeast in bread dough feeds on sugar to produce carbon dioxide. Why does the dough rise faster in a warmer area?
A)There is a greater number of effective collisions among reacting molecules.
B)Atmospheric pressure decreases with increasing temperature.
C)The yeast tends to "wake up" with warmer temperatures, which is why baker's yeast is best stored in the refrigerator.
D)The rate of evaporation increases with increasing temperature.
A)There is a greater number of effective collisions among reacting molecules.
B)Atmospheric pressure decreases with increasing temperature.
C)The yeast tends to "wake up" with warmer temperatures, which is why baker's yeast is best stored in the refrigerator.
D)The rate of evaporation increases with increasing temperature.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
58
For the above energy profiles, which reaction has the lowest activation energy?
A)a
B)b
C)c
D)d
E)All have the same activation energy.
A)a
B)b
C)c
D)d
E)All have the same activation energy.

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
59
What is the main difficulty in trying to eliminate chlorinated fluorocarbons from the atmosphere?
A)They are very stable and take a long time to decompose completely.
B)They are too light and blow away.
C)They do not absorb ultraviolet radiation.
D)all of the above
E)only a and b
A)They are very stable and take a long time to decompose completely.
B)They are too light and blow away.
C)They do not absorb ultraviolet radiation.
D)all of the above
E)only a and b

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
60
Why do the natural reactions involving ozone not lead to depletion of the ozone layer?
A)Ozone continually rises from the troposphere.
B)Ozone is too diffuse.
C)Ozone breaks into fragments that can reassemble into more ozone.
D)UV radiation generates more ozone.
E)c and d
A)Ozone continually rises from the troposphere.
B)Ozone is too diffuse.
C)Ozone breaks into fragments that can reassemble into more ozone.
D)UV radiation generates more ozone.
E)c and d

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
61
According to the following reaction, which molecule is acting as an acid? OH- + NH₄+ → H₂O + NH₃
A)H₂O
B)NH₃
C)OH-
D)NH₄+
E)none of the above
A)H₂O
B)NH₃
C)OH-
D)NH₄+
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
62
According to the following reaction, which molecule is acting as an acid? H₂O + H₂SO₄ → H₃O+ + HSO₄-
A)H₂SO₄
B)H₂O
C)H₃O+
D)HSO₄-
E)none of the above
A)H₂SO₄
B)H₂O
C)H₃O+
D)HSO₄-
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
63
How do you make a proton out of a hydrogen atom?
A)remove an electron from a hydrogen atom
B)remove a proton from a helium nucleus
C)let the hydrogen atoms undergo fusion
D)let the hydrogen atoms combine to form a hydrogen molecule and eject an electron
E)let the hydrogen atoms combine to form a hydrogen molecule and eject a proton
A)remove an electron from a hydrogen atom
B)remove a proton from a helium nucleus
C)let the hydrogen atoms undergo fusion
D)let the hydrogen atoms combine to form a hydrogen molecule and eject an electron
E)let the hydrogen atoms combine to form a hydrogen molecule and eject a proton

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
64
According to the following reaction, which molecule is acting as a base? H₂O + NH₃ → OH- + NH₄+
A)H₂O
B)NH₃
C)OH-
D)NH₄+
E)none of the above
A)H₂O
B)NH₃
C)OH-
D)NH₄+
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
65
According to the following reaction, which molecule is acting as an acid? H₂O + NH₃ → OH- + NH₄+
A)H₂O
B)NH₃
C)OH-
D)NH₄+
E)none of the above
A)H₂O
B)NH₃
C)OH-
D)NH₄+
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
66
According to the following reaction, which molecule is acting as a base? H₂O + H₂SO₄ → H₃O+ + HSO₄-
A)H₂SO₄
B)H₂O
C)H₃O+
D)HSO₄-
E)none of the above
A)H₂SO₄
B)H₂O
C)H₃O+
D)HSO₄-
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
67
Why is there a drastic reduction in ozone in the spring?
A)There is a sudden increase in Cl atoms in the spring.
B)It is warmer in the spring.
C)There are more chlorofluorocarbons in the spring.
D)all of the above
E)only a and b
A)There is a sudden increase in Cl atoms in the spring.
B)It is warmer in the spring.
C)There are more chlorofluorocarbons in the spring.
D)all of the above
E)only a and b

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following compounds would least likely act as an acid?
A)SO₄-2
B)HSO₄-1
C)H₂SO₄
D)NH₃
E)CH₃CO₂H
A)SO₄-2
B)HSO₄-1
C)H₂SO₄
D)NH₃
E)CH₃CO₂H

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
69
According to the following reaction, which molecule is acting as a base? H₃O+ + HSO₄- → H₂O + H₂SO₄
A)H₂SO₄
B)H₂O
C)H₃O+
D)HSO₄-
E)none of the above
A)H₂SO₄
B)H₂O
C)H₃O+
D)HSO₄-
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
70
The portion of the atmosphere that filters out 95 percent of the incident UV radiation is the ________.
A)ozone layer
B)stratosphere
C)troposphere
D)aerosol layer
E)particulates
A)ozone layer
B)stratosphere
C)troposphere
D)aerosol layer
E)particulates

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
71
Since some of the compounds that are destroying ozone are found in nature normally, how is it possible to tell that the reactive molecules destroying the ozone are due to our actions?
A)They are accompanied by compounds that are only man-made.
B)The compounds only increased in the past few years.
C)The isotope distribution is unique to man-made elements.
D)The chemical composition is different than natural compounds.
E)all of the above
A)They are accompanied by compounds that are only man-made.
B)The compounds only increased in the past few years.
C)The isotope distribution is unique to man-made elements.
D)The chemical composition is different than natural compounds.
E)all of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
72
Many people hear about atmospheric ozone depletion and wonder why we don't simply replace that which has been destroyed. Knowing about CFCs and how catalysts work, explain how this would not be a lasting solution.
A)The amount of energy required to create and transport sufficient ozone to the stratosphere would be cost prohibitive.
B)Governments would be too slow to respond as they argued about who should shoulder the burden of undertaking such an endeavor.
C)Any ozone we placed into the stratosphere would be destroyed by the same catalytic action that destroys naturally occurring stratospheric ozone.
D)all of the above
A)The amount of energy required to create and transport sufficient ozone to the stratosphere would be cost prohibitive.
B)Governments would be too slow to respond as they argued about who should shoulder the burden of undertaking such an endeavor.
C)Any ozone we placed into the stratosphere would be destroyed by the same catalytic action that destroys naturally occurring stratospheric ozone.
D)all of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
73
How is ozone produced in the upper atmosphere?
A)It forms by reaction of oxygen molecules with UV light.
B)It forms by nuclear decomposition of oxygen.
C)It diffuses from the lower atmosphere.
D)It forms by the reaction of nitric dioxide and oxygen.
E)all of the above
A)It forms by reaction of oxygen molecules with UV light.
B)It forms by nuclear decomposition of oxygen.
C)It diffuses from the lower atmosphere.
D)It forms by the reaction of nitric dioxide and oxygen.
E)all of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
74
Carefully examine the following reaction sequence for the catalytic formation of ozone, O₃, from molecular oxygen, O₂. Which chemical compound is behaving as the catalyst? O₂ + 2 NO → 2 NO₂
2 NO₂ → 2 NO + 2 O
2 O + 2 O₂ → 2 O₃
A)nitrogen monoxide, NO
B)nitrogen dioxide, NO₂
C)oxygen, O₂
D)atomic oxygen, O
2 NO₂ → 2 NO + 2 O
2 O + 2 O₂ → 2 O₃
A)nitrogen monoxide, NO
B)nitrogen dioxide, NO₂
C)oxygen, O₂
D)atomic oxygen, O

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
75
What is an acid?
A)anything that donates hydrogen ions
B)anything that accepts hydrogen atoms
C)anything that donates hydrogen atoms
D)anything that dissolves metal
E)anything that donates hydronium ions
A)anything that donates hydrogen ions
B)anything that accepts hydrogen atoms
C)anything that donates hydrogen atoms
D)anything that dissolves metal
E)anything that donates hydronium ions

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
76
What is a base?
A)anything that accepts a hydrogen ion
B)anything that accepts a hydroxide ion
C)anything that donates a hydroxide ion
D)anything that can be used to clean drains
E)anything with a bitter taste
A)anything that accepts a hydrogen ion
B)anything that accepts a hydroxide ion
C)anything that donates a hydroxide ion
D)anything that can be used to clean drains
E)anything with a bitter taste

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
77
The chlorinated fluorocarbons molecules are acting as a(n)________ by converting hundreds of thousands of molecules before they are inactivated.
A)catalyst
B)aerosol
C)active site
D)acid
E)base
A)catalyst
B)aerosol
C)active site
D)acid
E)base

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
78
According to the following reaction, which molecule is acting as an acid? H₃O+ + HSO₄- → H₂O + H₂SO₄
A)H₂SO₄
B)H₂O
C)H₃O+
D)HSO₄-
E)none of the above
A)H₂SO₄
B)H₂O
C)H₃O+
D)HSO₄-
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
79
What best describes what happens when an acid such as HCl is mixed with water?
A)The proton chemically bonded to the chlorine is transferred to a water molecule and forms a chloride ion and a hydronium ion.
B)A proton from the chlorine nucleus is ejected and captured by a water molecule to form a negatively charged HCl and a new hydronium ion.
C)A hydroxide ion from the water is transferred to the HCl molecule to form a proton and hydronium ion.
D)HCl is not an acid.
E)none of the above
A)The proton chemically bonded to the chlorine is transferred to a water molecule and forms a chloride ion and a hydronium ion.
B)A proton from the chlorine nucleus is ejected and captured by a water molecule to form a negatively charged HCl and a new hydronium ion.
C)A hydroxide ion from the water is transferred to the HCl molecule to form a proton and hydronium ion.
D)HCl is not an acid.
E)none of the above

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck
80
A catalytic converter increases the amount of carbon dioxide emitted by an automobile. Is this good news or bad news?
A)bad news because carbon dioxide contributes to global warming
B)good news because plants thrive on carbon dioxide
C)bad news because the carbon dioxide contributes to acid rain
D)good news because it means more noxious chemicals are not being emitted
A)bad news because carbon dioxide contributes to global warming
B)good news because plants thrive on carbon dioxide
C)bad news because the carbon dioxide contributes to acid rain
D)good news because it means more noxious chemicals are not being emitted

Unlock Deck
Unlock for access to all 252 flashcards in this deck.
Unlock Deck
k this deck


