Deck 3: Electromagnetic and Particulate Radiation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 3: Electromagnetic and Particulate Radiation
1
Different electromagnetic radiations are put in order by the
A) electromagnetic table
B) electromagnetic spectrum
C) electromagnetic chart
D) electromagnetic list
A) electromagnetic table
B) electromagnetic spectrum
C) electromagnetic chart
D) electromagnetic list
electromagnetic spectrum
2
In the formula E = hf, energy is measured in
A) volts
B) amps
C) frequency volts
D) electron volts
A) volts
B) amps
C) frequency volts
D) electron volts
electron volts
3
When considering the wave properties of electromagnetic radiation, the maximum height of a wave is
A) wavelength
B) amplitude
C) frequency
D) velocity
A) wavelength
B) amplitude
C) frequency
D) velocity
amplitude
4
In the formula E = hf, E represents
A) Planck's constant
B) frequency
C) energy
D) electricity
A) Planck's constant
B) frequency
C) energy
D) electricity

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Frequency is typically measured in
A) hertz
B) feet
C) centimeters
D) meters
A) hertz
B) feet
C) centimeters
D) meters

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
Electromagnetic theory was developed in the late 1800s by
A) Bohr
B) Rutherford
C) Maxwell
D) Planck
A) Bohr
B) Rutherford
C) Maxwell
D) Planck

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following electromagnetic radiations have the lowest energy level?
A) X-rays
B) Visible light
C) Microwaves
D) Radio waves
A) X-rays
B) Visible light
C) Microwaves
D) Radio waves

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
Electromagnetic radiation is a form of energy that originates from
A) electricity
B) magnetism
C) the atom
D) something moving in space
A) electricity
B) magnetism
C) the atom
D) something moving in space

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
The formula E = hf demonstrates that
A) as frequency increases, energy decreases
B) as frequency increases, energy increases
C) as energy increases, heat decreases
D) as energy increases, heat increases
A) as frequency increases, energy decreases
B) as frequency increases, energy increases
C) as energy increases, heat decreases
D) as energy increases, heat increases

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
As a wave's wavelength increases, its frequency
A) stays the same
B) increases
C) decreases
D) it depends on the type of electromagnetic radiation
A) stays the same
B) increases
C) decreases
D) it depends on the type of electromagnetic radiation

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
When considering the wave properties of electromagnetic radiation, the number of waves that pass by a given point per second is
A) wavelength
B) amplitude
C) frequency
D) velocity
A) wavelength
B) amplitude
C) frequency
D) velocity

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is not part of the electromagnetic spectrum?
A) Visible light
B) Microwaves
C) Sound
D) Gamma rays
A) Visible light
B) Microwaves
C) Sound
D) Gamma rays

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Microwaves travel at a velocity of
A) 3 * 106 m/s
B) 3 * 107 m/s
C) 3 *108 m/s
D) 3 * 109 m/s
A) 3 * 106 m/s
B) 3 * 107 m/s
C) 3 *108 m/s
D) 3 * 109 m/s

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
When considering the wave properties of electromagnetic radiation, the distance from the peak of one wave to the next is
A) wavelength
B) amplitude
C) frequency
D) velocity
A) wavelength
B) amplitude
C) frequency
D) velocity

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
What kind of disturbance does electromagnetic radiation NOT cause?
A) electrical
B) mechanical
C) magnetic
A) electrical
B) mechanical
C) magnetic

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
In the formula E = hf, f represents
A) Planck's constant
B) frequency
C) energy
D) electricity
A) Planck's constant
B) frequency
C) energy
D) electricity

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
Electromagnetic radiation
A) has no mass
B) has significant mass
C) has varying amounts of mass
D) none of these
A) has no mass
B) has significant mass
C) has varying amounts of mass
D) none of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
In the formula E = hf, h represents
A) Planck's constant
B) frequency
C) energy
D) electricity
A) Planck's constant
B) frequency
C) energy
D) electricity

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
Wavelength is generally measured in
A) hertz
B) feet
C) centimeters
D) meters
A) hertz
B) feet
C) centimeters
D) meters

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
As the wave's frequency increases, the wavelength
A) stays the same
B) increases
C) decreases
D) it depends on the type of electromagnetic radiation
A) stays the same
B) increases
C) decreases
D) it depends on the type of electromagnetic radiation

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
The process of removing an electron from an atom is
A) annihilation
B) atomization
C) ionization
D) none of these
A) annihilation
B) atomization
C) ionization
D) none of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
Gamma rays are produced
A) from unstable atoms
B) using fast-moving electrons
C) using fast-moving atoms
D) using fast-moving metals
A) from unstable atoms
B) using fast-moving electrons
C) using fast-moving atoms
D) using fast-moving metals

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
The inverse square law relates the intensity (of light/x-rays) to
A) velocity
B) time
C) mass
D) distance
A) velocity
B) time
C) mass
D) distance

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Microwaves heat up food because their energy causes the atoms and molecules to
A) dissolve
B) expand
C) vibrate
D) contract
A) dissolve
B) expand
C) vibrate
D) contract

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
An object we see as white is _______________ all of the wavelengths of visible light.
A) absorbing
B) diffusing
C) reflecting
D) changing
A) absorbing
B) diffusing
C) reflecting
D) changing

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
The electromagnetic radiation that passes between the television remote and television is
A) visible light
B) infrared light
C) microwaves
D) ultraviolet light
A) visible light
B) infrared light
C) microwaves
D) ultraviolet light

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
What is the frequency of electromagnetic radiation if the wavelength is 1 * 10-4 m?
A) 3 * 10-12 Hz.
B) 3 *10-4 Hz.
C) 3 *104 Hz.
D) 3 * 1012 Hz.
A) 3 * 10-12 Hz.
B) 3 *10-4 Hz.
C) 3 *104 Hz.
D) 3 * 1012 Hz.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
Particulate radiation does NOT include:
A) alpha particles
B) x-ray particles
C) beta particles
A) alpha particles
B) x-ray particles
C) beta particles

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Radio waves are used in
A) computed tomography
B) ultrasound
C) radiography
D) magnetic resonance imaging
A) computed tomography
B) ultrasound
C) radiography
D) magnetic resonance imaging

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
What characteristics of electromagnetic waves can the formula 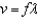 be used to calculate?
be used to calculate?
A) frequency and wavelength
B) frequency and velocity
C) velocity and wavelength
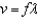 be used to calculate?
be used to calculate?A) frequency and wavelength
B) frequency and velocity
C) velocity and wavelength

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
When the formula 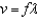 is changed to
is changed to 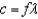 , the c represents the constant symbol for:
, the c represents the constant symbol for:
A) wavelength
B) frequency
C) amplitude
D) the speed of light
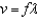 is changed to
is changed to 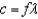 , the c represents the constant symbol for:
, the c represents the constant symbol for:A) wavelength
B) frequency
C) amplitude
D) the speed of light

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
An object we see as black is _______________ all of the wavelengths of visible light.
A) absorbing
B) diffusing
C) reflecting
D) changing
A) absorbing
B) diffusing
C) reflecting
D) changing

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
The electromagnetic radiation used in tanning beds is
A) visible light
B) infrared light
C) microwaves
D) ultraviolet light
A) visible light
B) infrared light
C) microwaves
D) ultraviolet light

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
The general process of a radioactive element giving off excess energy and particles to regain stability is
A) radioactivity
B) radioactive decay
C) ionization
D) electron emission
A) radioactivity
B) radioactive decay
C) ionization
D) electron emission

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
X-rays and gamma rays differ in
A) the energy source that produces them
B) the effect they have on matter
C) their energy level
D) all of these
A) the energy source that produces them
B) the effect they have on matter
C) their energy level
D) all of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
X-rays are produced
A) from unstable atoms
B) using fast-moving electrons
C) using fast-moving atoms
D) using fast-moving metals
A) from unstable atoms
B) using fast-moving electrons
C) using fast-moving atoms
D) using fast-moving metals

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
Alpha and beta particles are similar to x-rays and gamma rays in that they
A) have no mass
B) are part of the electromagnetic spectrum
C) have the energy to ionize matter
D) have characteristics of wavelength and frequency
A) have no mass
B) are part of the electromagnetic spectrum
C) have the energy to ionize matter
D) have characteristics of wavelength and frequency

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Radioactive elements
A) have excess energy in their nuclei
B) emit particles and energy
C) are trying to become stable elements
D) all of these
A) have excess energy in their nuclei
B) emit particles and energy
C) are trying to become stable elements
D) all of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
What is the wavelength of electromagnetic radiation if the frequency is 3 * 1010 m?
A) 1* 10-2 m.
B) 1 * 102 m.
C) 3 *10-2 m.
D) 3 * 102 m.
A) 1* 10-2 m.
B) 1 * 102 m.
C) 3 *10-2 m.
D) 3 * 102 m.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
An ion pair is
A) an electron and proton
B) a proton and neutron
C) an atom and the electron that was removed from it
D) an atom with an extra electron and an atom that is missing an electron
A) an electron and proton
B) a proton and neutron
C) an atom and the electron that was removed from it
D) an atom with an extra electron and an atom that is missing an electron

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
All of the electromagnetic radiations are capable of ionizing matter.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
Electromagnetic energy cannot travel through a vacuum.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
When the alpha particle picks up two electrons as it passes through air it becomes a
A) neutral atom of hydrogen
B) radioactive atom of hydrogen
C) neutral atom of helium
D) radioactive atom of helium
A) neutral atom of hydrogen
B) radioactive atom of hydrogen
C) neutral atom of helium
D) radioactive atom of helium

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
A negatively charged beta particle behaves the same as a(n)
A) proton
B) neutron
C) electron
D) positron
A) proton
B) neutron
C) electron
D) positron

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
The half-life of technetium-99m is 6 hours. How many unstable atoms will remain after 12 hours?
A) One sixth of the original amount.
B) One fourth of the original amount.
C) One third of the original amount.
D) Half of the original amount.
A) One sixth of the original amount.
B) One fourth of the original amount.
C) One third of the original amount.
D) Half of the original amount.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
Higher energy radiation acts more like waves than like particles.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
We are exposed to non-medical ionizing radiation everyday categorized as natural or background radiation.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
Half-life is
A) the rate at which a radioactive material decays
B) half the time it takes for all the radioactivity to decay
C) the rate at which particulate radiation is emitted from a radioactive atom's nucleus
D) none of these
A) the rate at which a radioactive material decays
B) half the time it takes for all the radioactivity to decay
C) the rate at which particulate radiation is emitted from a radioactive atom's nucleus
D) none of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
The beta particle is an electron that is emitted from an outer shell.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
All electromagnetic radiation travels at the same speed.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
Electromagnetic radiation has properties of a wave and a particle.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
As compared to an alpha particle, a beta particle
A) has less mass
B) has more mass
C) has the same mass
D) is less penetrating
A) has less mass
B) has more mass
C) has the same mass
D) is less penetrating

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
An alpha particle is the same as the nucleus of a(n)
A) hydrogen atom
B) oxygen atom
C) carbon atom
D) helium atom
A) hydrogen atom
B) oxygen atom
C) carbon atom
D) helium atom

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is NOT a source of manmade ionizing radiation?
A) x-rays
B) radiopharmaceuticals used in nuclear medicine
C) gamma rays
D) all are mand made ionizing radiation
A) x-rays
B) radiopharmaceuticals used in nuclear medicine
C) gamma rays
D) all are mand made ionizing radiation

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
The positively charged beta particle is a(n)
A) electron
B) alpha particle
C) negatron
D) positron
A) electron
B) alpha particle
C) negatron
D) positron

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
When compared with a beta particle, the alpha particle is
A) much smaller
B) much larger
C) more penetrating
D) none of these
A) much smaller
B) much larger
C) more penetrating
D) none of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
All radioactive elements occur naturally.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
Alpha particles consist of
A) two protons and two electrons
B) two protons and two neutrons
C) two electrons and two neutrons
D) none of these
A) two protons and two electrons
B) two protons and two neutrons
C) two electrons and two neutrons
D) none of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
X-rays and gamma rays are the only electromagnetic radiations that are able to ionize matter.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
Half-life is defined as
A) half the time it takes for all of the remaining atoms in an amount of a radioactive element to decay
B) half the time it takes for half the remaining atoms in an amount of a radioactive element to decay
C) the time it takes for half the remaining atoms in an amount of a radioactive element to decay
D) none of these
A) half the time it takes for all of the remaining atoms in an amount of a radioactive element to decay
B) half the time it takes for half the remaining atoms in an amount of a radioactive element to decay
C) the time it takes for half the remaining atoms in an amount of a radioactive element to decay
D) none of these

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


