Deck 6: Properties of Solutions I: Aqueous Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 6: Properties of Solutions I: Aqueous Solutions
1
Which of the following is the correct name for the acid H3PO4?
A) hydrophosphoric acid
B) phosphoric acid
C) phosphate acid
D) phosphorous acid
A) hydrophosphoric acid
B) phosphoric acid
C) phosphate acid
D) phosphorous acid
phosphoric acid
2
A 25.0-mL solution contains 0.45 g of cadmium cyanide. Which type of solution is this?
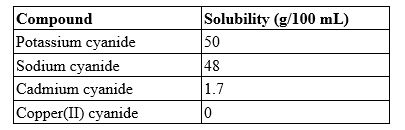
A) saturated
B) supersaturated
C) undersaturated
D) unsaturated

A) saturated
B) supersaturated
C) undersaturated
D) unsaturated
supersaturated
3
A luminol solution is used to detect the presence of blood. What is the molarity of a luminol solution that was made by dissolving 15.5 g of luminol (C8H7N3O2) in water to a final volume of 90.0 mL?
A) 0.0875 M
B) 0.972 M
C) 0.172 M
D) 0.00787 M
A) 0.0875 M
B) 0.972 M
C) 0.172 M
D) 0.00787 M
0.972 M
4
Which of the following compounds is soluble in water?
A) silver chloride
B) barium carbonate
C) calcium phosphate
D) potassium hydroxide
A) silver chloride
B) barium carbonate
C) calcium phosphate
D) potassium hydroxide

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
An unknown solution is determined to have a pH of 4.5. Identify the solution as being acidic, basic, or neutral.
A) acidic
B) basic
C) neutral
A) acidic
B) basic
C) neutral

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
What is the pH of a 1.4 M HCl solution?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Write the net ionic equation for the precipitation of lead(II) iodide from the addition of lead(II) nitrate solution to a potassium iodide solution.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compound is a weak electrolyte?
A) HBr
B) Sr(OH)2
C) NaCl
D) H3PO4
A) HBr
B) Sr(OH)2
C) NaCl
D) H3PO4

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
Identify Ca(OH)2 as a strong acid, strong base, weak acid, or weak base.
A) strong acid
B) strong base
C) weak acid
D) weak base
A) strong acid
B) strong base
C) weak acid
D) weak base

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Identify H2CO3 as a strong acid, strong base, weak acid, or weak base.
A) strong acid
B) strong base
C) weak acid
D) weak base
A) strong acid
B) strong base
C) weak acid
D) weak base

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compound is insoluble in water?
A) Na3PO4
B) CaS
C) PbSO4
D) (NH4)2CrO4
A) Na3PO4
B) CaS
C) PbSO4
D) (NH4)2CrO4

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
What is the molarity of a solution prepared by dissolving 29.3 g KCl in water to a final volume of 500.0 mL?
A) 0.586 M
B) 58.6 M
C) 0.786 M
D) 0.000786 M
A) 0.586 M
B) 58.6 M
C) 0.786 M
D) 0.000786 M

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds is a strong electrolyte?
A) HF
B) NH3
C) NaOH
D) CH3CO2H
Enter the appropriate word(s) to complete the statement.
A) HF
B) NH3
C) NaOH
D) CH3CO2H
Enter the appropriate word(s) to complete the statement.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Which term is used to describe a solution that contains less solute than the maximum amount of solute that can be dissolved?
A) saturated
B) supersaturated
C) undersaturated
D) unsaturated
A) saturated
B) supersaturated
C) undersaturated
D) unsaturated

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
State whether each of the following compounds is an electrolyte or nonelectrolyte.
A) CaCl2 ______
B) CO2 _____
C) H2SO4 _____
D) C6H12O6 _____
A) CaCl2 ______
B) CO2 _____
C) H2SO4 _____
D) C6H12O6 _____

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
Triclosan is an antibacterial agent present in some soaps and surgical cleaning treatments. Calculate the mass of triclosan, C12H7Cl3O2, needed to prepare 750.0 mL of a 5.00 × 10-5 M solution.
A) 0.109 g
B) 0.0109 g
C) 3.75 × 10-5 g
D) 6.67 × 10-5 g
A) 0.109 g
B) 0.0109 g
C) 3.75 × 10-5 g
D) 6.67 × 10-5 g

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
A 100.0-mL solution contains 27.8 g of sodium cyanide. Which type of solution is this?
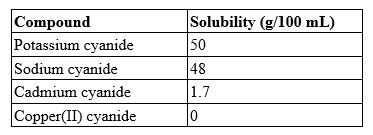
A) saturated
B) supersaturated
C) undersaturated
D) unsaturated

A) saturated
B) supersaturated
C) undersaturated
D) unsaturated

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
Hydrofluoric acid is used to etch glass. Concentrated hydrofluoric acid is 28.9 M. If a 500.0-mL sample of 5.75 M HF is needed, how many mL of the concentrated HF should be diluted?
A) 0.0100 mL
B) 83.1 mL
C) 99.5 mL
D) 2510 mL
A) 0.0100 mL
B) 83.1 mL
C) 99.5 mL
D) 2510 mL

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
What ion would be used to form an insoluble compound with the ion Hg22+?
A) S2-
B) C2H3O2-
C) NO3-
D) Br-
A) S2-
B) C2H3O2-
C) NO3-
D) Br-

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
What is the pH of a 0.280 M HNO3 solution?

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


