Deck 5: Chemistry of Bonding:
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 5: Chemistry of Bonding:
1
Select the molecular geometry of phosphorous trihydride, PH3, an insecticide.
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
trigonal pyramidal
2
Determine the electron geometry of the tin(IV) chloride, SnCl4, used as a catalyst in organic synthesis.
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
tetrahedral
3
Select the electron geometry of boron trichloride, BCl3, used to refine aluminum.
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
trigonal planar
4
Draw the Lewis dot structure for the ionic bonding in Al2S3.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
Indicate whether the following molecules are polar or nonpolar.
NH3 _____
CH4 _____
CO2 _____
H2S _____
NH3 _____
CH4 _____
CO2 _____
H2S _____

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
What is the molecular geometry for carbon 1? 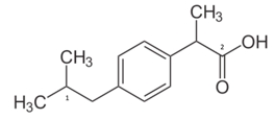
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
Select the molecular geometry for the following Lewis structure: 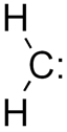
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
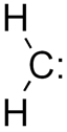
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
Draw the Lewis dot structure for the ionic bonding in NaBr.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
Draw the Lewis dot structure for the element B.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Select the molecular geometry for the following Lewis structure: 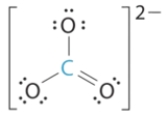
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Draw the Lewis structure for the covalent bonding in CN-.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
Draw the Lewis dot structure for the element I.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
Select the molecular geometry of carbon disulfide, CS2, used in the manufacturing of cellophane.
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
Draw the Lewis structures representing the resonance structures of ozone, O3.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
Draw three of the seven possible Lewis structures representing the resonance structures of SO3.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
What is the molecular geometry for carbon 2? 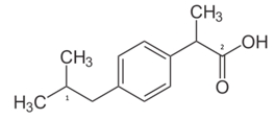
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
Draw the Lewis structure for the covalent bonding in BCl3.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
How does cocaine interfere with the uptake of neurotransmitters?
A) Cocaine blocks neurotransmitters from reentering a cell.
B) Cocaine binds with neurons and creates a new compound in the cells.
C) Cocaine destroys neurons so they can no longer send electrical signals.
D) Cocaine has its own action potential that interferes with the action potential that neurons send.
A) Cocaine blocks neurotransmitters from reentering a cell.
B) Cocaine binds with neurons and creates a new compound in the cells.
C) Cocaine destroys neurons so they can no longer send electrical signals.
D) Cocaine has its own action potential that interferes with the action potential that neurons send.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
Select the electron geometry of iodine in iodine monochloride, ICl, used in organic synthesis.
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal
A) bent
B) linear
C) tetrahedral
D) trigonal planar
E) trigonal pyramidal

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
Draw the overlap between p atomic orbitals to show the covalent bond between the Cl atoms in chlorine gas (Cl2)

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


