Deck 6: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 6: Gases
1
The SI unit of pressure is atmosphereis defined as exactly 760 mmHg. (atm).
False
2
Avogadro's law states that equal volumes of different gases at the same temperature and pressure contain the same amount of gas.
True
3
Gay-Lussac's law relates volume of a gas with absolute temperature.
False
4
When applying Charles's law, amount of gas and volume are considered to be constants.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
A sample of a gas has an initial volume of 15.0 L and an initial temperature of 235 K. The temperature of the gas increases to 313 K, if its volume increases to 20.0 L.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
P1T1 = P2T2, represents the Charles's law of gases.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
Real gases are ideal.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
One torr is an equivalent unit of one millimeter of mercury, the amount of pressure exerted by a column of mercury exactly 1 mm high.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
According to Gay-Lussac's law, 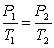 at constant V and n.
at constant V and n.
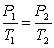 at constant V and n.
at constant V and n.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
A 6.00 mol sample of O2 has a pressure of 0.80 atm and a temperature of 68°C. Its volume is 41.8L.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
Robert Boyle invented the mercury barometer.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
The density of O2 is 1.54 g/L at 30°C and 1.20 atm.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
If a 2.45 L volume of gas contains 4.5 × 1021 gas particles, a 4.9 L volume of gas will contain 4.5 × 1022 gas particles.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
An ideal gas is a gas that exactly follows the statements of the kinetic theory.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
Pressure and volume of a gas are inversely related, if other parameters are kept the same.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
Pascal (Pa) is equal to 1 N/m2.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
A sample of a gas has an initial volume of 16.8 L and an initial temperature of 50°C. The volume of the gas increases to 33.6 L if its temperature is increased to 100° C.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
The equation, PV = nRT is called the ideal gas law, a gas law that relates all four independent physical properties of a gas under any conditions..

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
According to the kinetic theory of gases, the size of a gas particle is large compared to the distances that separate them.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
The combined gas law considers the amount of gas as a variable.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
A gas has an initial pressure of 1.823 atm and an initial volume of 8.026 L. What is its new pressure if volume is changed to 6.323 L? Assume temperature and amount of gas are held constant.
A) 0.9342 atm
B) 1.436 atm
C) 3.105 atm
D) 2.314 atm
E) 27.84 atm
A) 0.9342 atm
B) 1.436 atm
C) 3.105 atm
D) 2.314 atm
E) 27.84 atm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
What is the Kelvin equivalent of 145°C?
A) 752
B) 293
C) 418
D) 116
E) 292
A) 752
B) 293
C) 418
D) 116
E) 292

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following laws states 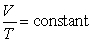 for a given amount of a gas, when pressure is constant?
for a given amount of a gas, when pressure is constant?
A) Charles's law
B) Boyle's law
C) Avogadro's law
D) Graham's law
E) Gay-Lussac's law
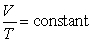 for a given amount of a gas, when pressure is constant?
for a given amount of a gas, when pressure is constant?A) Charles's law
B) Boyle's law
C) Avogadro's law
D) Graham's law
E) Gay-Lussac's law

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
is a gas that deviates slightly from agreeing perfectly with the kinetic theory of gases. X is an example of _____ gas.
A) real
B) ideal
C) supreme
D) static
E) sturdy
A) real
B) ideal
C) supreme
D) static
E) sturdy

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
Pressure at a point deep in an ocean is estimated to be 1.074 × 103 kPa. What is the average atmospheric pressure at that point?
A) 1.074 × 10-1 atm
B) 1.060 × 106 atm
C) 1.060 x 101 atm
D) 1.074 × 10-4 atm
E) 1.074 × 103 atm
A) 1.074 × 10-1 atm
B) 1.060 × 106 atm
C) 1.060 x 101 atm
D) 1.074 × 10-4 atm
E) 1.074 × 103 atm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
Boyle's law explains the relationship between _____.
A) pressure and temperature
B) volume and temperature
C) the amount of gas and temperature
D) pressure and the amount of gas
E) pressure and volume
A) pressure and temperature
B) volume and temperature
C) the amount of gas and temperature
D) pressure and the amount of gas
E) pressure and volume

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
The vapor pressure of water increases with an increase in its temperature.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements is true about the relationship between pressure and volume?
A) Pressure and volume are independent of each other.
B) Pressure and volume decrease with an increase in temperature.
C) Pressure increases as volume decreases.
D) Pressure and volume are not related to temperature.
E) Pressure increases as volume increases.
A) Pressure and volume are independent of each other.
B) Pressure and volume decrease with an increase in temperature.
C) Pressure increases as volume decreases.
D) Pressure and volume are not related to temperature.
E) Pressure increases as volume increases.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
Experiments show that the volume of a gas is related to its temperature in _____.
A) Celsius
B) Fahrenheit
C) Kelvin
D) Joule
E) Newton
A) Celsius
B) Fahrenheit
C) Kelvin
D) Joule
E) Newton

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
If mixture of H2 at 5.00 atm and N2 at 8.00 atm is stored in a container, the mixture's pressure will be 10.1 atm.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
A gas has an initial pressure of 1,080 torr and an initial volume of 586 mL. What is its new volume if pressure is changed to 1.30 atm? Assume temperature and amount of gas are held constant.
A) 370 mL
B) 486 L
C) 641 L
D) 487 L
E) 641 mL
A) 370 mL
B) 486 L
C) 641 L
D) 487 L
E) 641 mL

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
If a force of 1.50 x103 N is pressed against an area of 3.00 m2, what is the pressure in torr?
A) 3.75
B) 5.65
C) 500
D) 565
E) 3,800
A) 3.75
B) 5.65
C) 500
D) 565
E) 3,800

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
A gas that exactly follows the statements of the kinetic theory is called _____.
A) real gas
B) kinetic gas
C) dynamic gas
D) ideal gas
E) stationary gas
A) real gas
B) kinetic gas
C) dynamic gas
D) ideal gas
E) stationary gas

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
How many atmospheres are there in 2,326 torr?
A) 2.370
B) 23.26
C) 11.63
D) 3.060
E) 1.160
A) 2.370
B) 23.26
C) 11.63
D) 3.060
E) 1.160

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
A sample of gas has an initial volume of 5.00 L and an initial temperature of 360. K. What is the new volume if the temperature is increased to 900. K? Assume constant pressure and amount for the gas.
A) 2.00 L
B) 3.00 L
C) 8.00 L
D) 12.5 L
E) 5.00 L
A) 2.00 L
B) 3.00 L
C) 8.00 L
D) 12.5 L
E) 5.00 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following properties of gases must be held constant when applying Boyle's law?
A) Pressure
B) Amount of gas
C) Volume
D) Shape of the container
E) Size of the container
A) Pressure
B) Amount of gas
C) Volume
D) Shape of the container
E) Size of the container

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is the SI approved unit of pressure?
A) Pounds/inch
B) Millimeters of mercury
C) Atmosphere
D) N/cm2
E) Pascal
A) Pounds/inch
B) Millimeters of mercury
C) Atmosphere
D) N/cm2
E) Pascal

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
_____ refers to the force of all the gas particle/wall collisions divided by the area of the wall.
A) Temperature
B) Pressure
C) HYPERLINK "http://en.wikipedia.org/wiki/Buoyancy" Buoyancy
D) Density
E) Concentration
A) Temperature
B) Pressure
C) HYPERLINK "http://en.wikipedia.org/wiki/Buoyancy" Buoyancy
D) Density
E) Concentration

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
The mole fraction of He is 0.43 in a mixture containing He at 0.60 atm and Ne at 0.80 atm.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following statements is consistent with the kinetic theory of gases?
A) Gases consist of tiny stationary particles of matter.
B) Gases constantly collide with each other.
C) The size of a gas particle is large when compared to the distances between them.
D) A net loss of energy occurs when particles collide.
E) Strong interactive forces exist between the particles of a gas.
A) Gases consist of tiny stationary particles of matter.
B) Gases constantly collide with each other.
C) The size of a gas particle is large when compared to the distances between them.
D) A net loss of energy occurs when particles collide.
E) Strong interactive forces exist between the particles of a gas.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
A sample of gas reduces its pressure and absolute temperature by 55 percent. What is the percentage change in volume?
A) 0
B) 20
C) 40
D) 50
E) 100
A) 0
B) 20
C) 40
D) 50
E) 100

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following gas laws relates pressure with absolute temperature?
A) Avogadro's law
B) Graham's law
C) Charles's law
D) Boyle's law
E) Gay-Lussac's law
A) Avogadro's law
B) Graham's law
C) Charles's law
D) Boyle's law
E) Gay-Lussac's law

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
A 1.6608 mol sample of O2 has a pressure of 0.9561 atm and a temperature of 386 K. What is its volume?
A) 55.0 L
B) 59.0 L
C) 30.2 L
D) 50.6 L
E) 59.8 L
A) 55.0 L
B) 59.0 L
C) 30.2 L
D) 50.6 L
E) 59.8 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is considered the standard temperature under STP?
A) 0 K
B) 273 K
C) 160 K
D) 300 K
E) 313 K
A) 0 K
B) 273 K
C) 160 K
D) 300 K
E) 313 K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
A sample of gas has an initial volume of 87.01 mL and an initial temperature of 295.5 K. What is the new temperature if the volume is decreased to 50.00 mL? Assume constant pressure and amount for the gas.
A) 169.8 K
B) 514.2 K
C) 158.6 K
D) 530.5 K
E) 14.72 K
A) 169.8 K
B) 514.2 K
C) 158.6 K
D) 530.5 K
E) 14.72 K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
How many moles of H2 are present in 69.2 L at STP?
A) 17.3 mol
B) 34.6 mol
C) 3.09 mol
D) 4.00 mol
E) 8.65 mol
A) 17.3 mol
B) 34.6 mol
C) 3.09 mol
D) 4.00 mol
E) 8.65 mol

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
A sample of gas has a volume of 60. mL and an initial temperature of 380 K. The temperature must be changed to _____ in order to halve the volume. Assume constant pressure and amount for the gas.
A) 273 K
B) 76 K
C) 190 K
D) 760 K
E) 780 K
A) 273 K
B) 76 K
C) 190 K
D) 760 K
E) 780 K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
A sample of gas at an initial volume of 65.0 mL, an initial pressure of 866 torr, and an initial temperature of 10.0°C simultaneously changes its temperature to 60.0°C and its volume to 46.0 mL. What is the final pressure of the gas?
A) 154 torr
B) 2,150 torr
C) 6,210 torr
D) 168 torr
E) 1,440 torr
A) 154 torr
B) 2,150 torr
C) 6,210 torr
D) 168 torr
E) 1,440 torr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following equations represent the ideal gas law?
A) P1V1 = P2V2
B)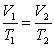
C)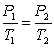
D)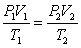
E) PV = nRT
A) P1V1 = P2V2
B)
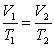
C)
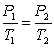
D)
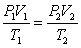
E) PV = nRT

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
A 5.86 mol sample of Ne has a pressure of 263 torr and a temperature of 54.0°C. What is its volume?
A) 0.598 L
B) 455 L
C) 0.0988 L
D) 460 L
E) 58.6 L
A) 0.598 L
B) 455 L
C) 0.0988 L
D) 460 L
E) 58.6 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following laws consider amount of gas as a variable?
A) Charles's law
B) Avogadro's law
C) Combined gas law
D) Boyle's law
E) Gay-Lussac's law
A) Charles's law
B) Avogadro's law
C) Combined gas law
D) Boyle's law
E) Gay-Lussac's law

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
What is the molar volume of oxygen gas (O2) at STP?
A) 44.8 L
B) 22.4 L
C) 103.04 L
D) 411.2 L
E) 716.8 L
A) 44.8 L
B) 22.4 L
C) 103.04 L
D) 411.2 L
E) 716.8 L

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following statements is true about the ideal gas law?
A) Pressure is considered to be a constant when applying the ideal gas law.
B) The ideal gas law does not require a change in the conditions of a gas sample.
C) Volume is considered to be a constant when applying the ideal gas law.
D) According to ideal gas law, temperature is not an independent property of gases.
E) The ideal gas law is represented by the equation P1V1 = P2V2.
A) Pressure is considered to be a constant when applying the ideal gas law.
B) The ideal gas law does not require a change in the conditions of a gas sample.
C) Volume is considered to be a constant when applying the ideal gas law.
D) According to ideal gas law, temperature is not an independent property of gases.
E) The ideal gas law is represented by the equation P1V1 = P2V2.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
What is the final temperature of a gas whose initial pressure is 455 torr, initial temperature is 105°C, and final pressure is 690 torr? Assume volume and amount of gas are held constant.
A) 300.°C
B) 69°C
C) 159°C
D) -23.7°C
E) 186°C
A) 300.°C
B) 69°C
C) 159°C
D) -23.7°C
E) 186°C

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
What is the final pressure of a gas whose initial pressure is 590 torr, initial temperature is 308 K, and final temperature is 286 K? Assume volume and amount of gas are held constant.
A) 585 torr
B) 386 torr
C) 150 torr
D) 550 torr
E) 631 torr
A) 585 torr
B) 386 torr
C) 150 torr
D) 550 torr
E) 631 torr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
A gas has an initial volume of 65.08 mL and an initial temperature of 577°C. What is its new volume if temperature is changed to 417°C? Assume pressure and amount of gas are held constant.
A) 56.2 mL
B) 47.0 mL
C) 80.2 mL
D) 90.1 mL
E) 52.8 mL
A) 56.2 mL
B) 47.0 mL
C) 80.2 mL
D) 90.1 mL
E) 52.8 mL

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is a constant when applying the combined gas law?
A) Pressure
B) Volume
C) Amount of gas
D) Temperature
E) Density
A) Pressure
B) Volume
C) Amount of gas
D) Temperature
E) Density

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
For a 0.0962 mol sample of O2, V = 58 L and T = 456 K. What is its pressure?
A) 0.61 atm
B) 0.021 atm
C) 0.21 atm
D) 0.047 atm
E) 0.062 atm
A) 0.61 atm
B) 0.021 atm
C) 0.21 atm
D) 0.047 atm
E) 0.062 atm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
A 3.8 mL volume of gas contains 5.6 × 1022 gas particles. How many gas particles are there in 15 mL if the gas is at the same pressure and temperature?
A) 3.8 × 1022
B) 1.5 × 1023
C) 2.2 × 1023
D) 1.5 × 1022
E) 5.4 × 1022
A) 3.8 × 1022
B) 1.5 × 1023
C) 2.2 × 1023
D) 1.5 × 1022
E) 5.4 × 1022

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following laws proposes a relationship between pressure, temperature, and volume of a gas when the amount of gas is a constant?
A) Gay-Lussac's law
B) Boyle's law
C) Combined gas law
D) Charles's law
E) Avogadro's law
A) Gay-Lussac's law
B) Boyle's law
C) Combined gas law
D) Charles's law
E) Avogadro's law

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
What is Avogadro's law? Explain.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
What is pressure? How is it calculated?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
Explain the concept of ideal gas law.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
A container has a mixture of H2 at 1.2 atm and O2 at 1.6 atm. What is the mole fraction of H2?
A) 0.55
B) 0.45
C) 0.57
D) 0.40
E) 0.43
A) 0.55
B) 0.45
C) 0.57
D) 0.40
E) 0.43

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following statements is consistent with the Dalton's law of partial pressures?
A) The total pressure of a mixture of gases is less than the sum of the partial pressures of individual gases.
B) The total pressure of a gas mixture is equal to the sum of the partial pressures of the components.
C) The total pressure of a gas mixture is calculated by assessing the weighted average of the molecular pressures of the components.
D) The total pressure of a gas mixture is independent of the partial pressures of the components.
E) The total pressure of a mixture of gases is more than the sum of the partial pressures of individual gases.
A) The total pressure of a mixture of gases is less than the sum of the partial pressures of individual gases.
B) The total pressure of a gas mixture is equal to the sum of the partial pressures of the components.
C) The total pressure of a gas mixture is calculated by assessing the weighted average of the molecular pressures of the components.
D) The total pressure of a gas mixture is independent of the partial pressures of the components.
E) The total pressure of a mixture of gases is more than the sum of the partial pressures of individual gases.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
Briefly explain Dalton's law of partial pressures.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
What are the postulates of the kinetic theory of gases?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
What is molar volume? What is the molar volume of gases at STP

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
Briefly explain Boyle's law.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
What is the combined gas law?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
Briefly describe Gay-Lussac's law. Highlight the difference between Gay-Lussac's and Charles's law.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
A mixture of helium at 1.600 atm and neon at 900.0 torr is in a container. What is the total pressure in the container?
A) 1,008 torr
B) 901.6 torr
C) 450.8 torr
D) 2,116 torr
E) 598 torr
A) 1,008 torr
B) 901.6 torr
C) 450.8 torr
D) 2,116 torr
E) 598 torr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
What are ideal gases? Can real world gases be called ideal gases?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
Briefly explain Charles's law.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
Explain the concept of standard temperature and pressure.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
Explain the significance and use of the ideal gas law.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
H2 stored in a 50.0 L container has a pressure of 500.0 torr. O2 stored in a 25.0 L container has a pressure of 800.0 torr. If both these gases are released together to be stored in a 100.0 L container, what is the total pressure in the 100.0 L container?
A) 800 torr
B) 500 torr
C) 450 torr
D) 650 torr
E) 1,300 torr
A) 800 torr
B) 500 torr
C) 450 torr
D) 650 torr
E) 1,300 torr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
CO2 generated by the decomposition of ZnCO3, is collected in a 5.6 L container over water. If the temperature is 40.0°C and the total pressure inside the container is 580 torr, how many moles of CO2 were generated?
A) 0.09 mol
B) 0.74 mol
C) 0.18 mol
D) 0.15 mol
E) 0.86 mol
A) 0.09 mol
B) 0.74 mol
C) 0.18 mol
D) 0.15 mol
E) 0.86 mol

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
What are the various units of pressure? Show how to convert one pressure unit into another using dimensional analysis.
The formal, SI-approved unit of pressure is the pascal (Pa), which is defined as 1 N/m2. A common unit of pressure is the atmosphere (atm), which was originally defined as the average atmospheric pressure at sea level. A more reliable and common unit is millimeters of mercury (mmHg), which is the amount of pressure exerted by a column of mercury exactly 1 mm high. An equivalent unit is the torr, which equals 1 mmHg. 1.00 atm
1.00 atm
The formal, SI-approved unit of pressure is the pascal (Pa), which is defined as 1 N/m2. A common unit of pressure is the atmosphere (atm), which was originally defined as the average atmospheric pressure at sea level. A more reliable and common unit is millimeters of mercury (mmHg), which is the amount of pressure exerted by a column of mercury exactly 1 mm high. An equivalent unit is the torr, which equals 1 mmHg.
 1.00 atm
1.00 atm
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
A mixture of H2 at 566 torr and O2 at 600 torr is in a container. What is the total pressure in the container?
A) 3,396 torr
B) 566 torr
C) 600 torr
D) 1,166 torr
E) 3,000 torr
A) 3,396 torr
B) 566 torr
C) 600 torr
D) 1,166 torr
E) 3,000 torr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck


