Deck 3: Atoms, Molecules, and Ions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 3: Atoms, Molecules, and Ions
1
According to chemical nomenclature, SO2 is referred to as sulfur dioxide.
True
2
I− is an example of a monatomic anion.
True
3
Atoms of the same element with different numbers of electrons are called isotopes.
False
4
'Au' is the atomic symbol of the element silver.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
The proton is a heavier subatomic particle than the electron and has a positive charge.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
The molecule Se2Br2 is called diselenide dibromide.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
Monatomic ions are formed when an atom loses or gains one or more electrons to attain a charge.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
The connection between two atoms in a molecule is called a chemical bond.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
According to the nomenclature of ionic compounds, MgCl2 is called magnesium dichloride.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
Hydrogen and oxygen exist naturally as three-atom molecules.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
N3− is called trinitride ion.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
The atomic mass unit is defined as one-twelfth of the mass of a nitrogen-14 atom.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
The molecular mass of dinitrogen trioxide is determined by adding the atomic mass of nitrogen two times with the atomic mass of oxygen three times.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
Species with overall negative charges are termed anions.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
Uranium-235 isotope of uranium has 143 neutrons.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
In the ionic compound NaCl, sodium ion is the cation and chloride ion is the anion.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
A proper covalent formula has a cation and an anion in it.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
The atomic mass of an element is a weighted average of the masses of the isobars of an atom.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
The number of protons and electrons in an element's nucleus is called the atomic number of the element.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
The smallest piece of an element that maintains the identity of that element is called a molecule.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
If a compound is composed of hydrogen ions and a polyatomic anion, then the name of the acid is derived from the stem of the polyatomic ion's name.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
Hg is the atomic symbol of the element _____.
A) nickel
B) helium
C) gallium
D) mercury
E) germanium
A) nickel
B) helium
C) gallium
D) mercury
E) germanium

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements is consistent with the modern atomic theory?
A) Atoms of different elements can have the same configuration.
B) Atoms combine in whole number ratios to form compounds.
C) All matter is composed of isotopes.
D) Atoms of the same element are not the same always.
E) An electron is smallest piece of an element that maintains its identity.
A) Atoms of different elements can have the same configuration.
B) Atoms combine in whole number ratios to form compounds.
C) All matter is composed of isotopes.
D) Atoms of the same element are not the same always.
E) An electron is smallest piece of an element that maintains its identity.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
The _____ is a relatively massive subatomic particle with a positive charge.
A) isotope
B) proton
C) neutron
D) nucleus
E) electron
A) isotope
B) proton
C) neutron
D) nucleus
E) electron

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
The smallest piece of an element that maintains the identity of that element is called a(n) _____.
A) compound
B) molecule
C) atom
D) ingredient
E) particle
A) compound
B) molecule
C) atom
D) ingredient
E) particle

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
Identify the subatomic particle with about the same mass as a proton but no charge.
A) Neutron
B) Nucleus
C) Electron
D) Isotope
E) Isobar
A) Neutron
B) Nucleus
C) Electron
D) Isotope
E) Isobar

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is an atom that is not a diatomic molecule?
A) Helium
B) Hydrogen
C) Chlorine
D) Fluorine
E) Oxygen
A) Helium
B) Hydrogen
C) Chlorine
D) Fluorine
E) Oxygen

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is a tiny subatomic particle with a negative charge?
A) Electron
B) Proton
C) Neutron
D) Photon
E) Isotope
A) Electron
B) Proton
C) Neutron
D) Photon
E) Isotope

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Atoms that contain more than one unit charge are called polyatomic ions.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
An acid is an ionic compound of the H+ cation dissolved in water.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
What is the approximate mass of a neutron, in kg?
A) 6.6 × 10−27
B) 9.1× 10−28
C) 1.6 × 10−27
D) 9.1 × 10−31
E) 1.6 × 10−31
A) 6.6 × 10−27
B) 9.1× 10−28
C) 1.6 × 10−27
D) 9.1 × 10−31
E) 1.6 × 10−31

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
Two atoms that have the same number of protons with different numbers of neutrons are called _____.
A) nuclear isomers
B) isotopes
C) isotones
D) isobars
E) chemical isobars
A) nuclear isomers
B) isotopes
C) isotones
D) isobars
E) chemical isobars

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
HCl is the formula for chloric acid.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
The number of protons in the nucleus of an atom is 80, while the number of neutrons in the nucleus is 90. What is the mass number of this isotope?
A) 10
B) 170
C) 80
D) 90
E) 135
A) 10
B) 170
C) 80
D) 90
E) 135

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following combinations refer to an isotope?
A)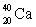 and
and 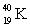
B)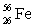 and
and 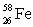
C)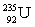 and
and 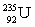
D)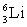 and
and 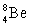
E)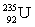 and
and 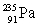
A)
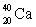 and
and 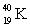
B)
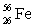 and
and 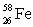
C)
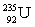 and
and 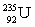
D)
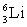 and
and 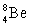
E)
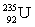 and
and 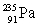

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
The sum of the number of protons and neutrons in a nucleus is referred to as the _____ number.
A) potential
B) elemental
C) atomic
D) magnetic
E) mass
A) potential
B) elemental
C) atomic
D) magnetic
E) mass

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
Fe2S3 is called iron(II) sulfide.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is the atomic symbol of gold?
A) Gl
B) Gd
C) An
D) Ga
E) Au
A) Gl
B) Gd
C) An
D) Ga
E) Au

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
The nucleus is made up of what two subatomic particles?
A) Proton and electron
B) Proton and neutron
C) Neutron and electron
D) Photon and electron
E) Photon and neutron
A) Proton and electron
B) Proton and neutron
C) Neutron and electron
D) Photon and electron
E) Photon and neutron

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
The atomic number of an atom refers to the _____.
A) number of protons in the atom
B) the atomic mass of the nucleus
C) the total mass of the electrons
D) number of neutrons in the atom
E) the atomic mass of the atom
A) number of protons in the atom
B) the atomic mass of the nucleus
C) the total mass of the electrons
D) number of neutrons in the atom
E) the atomic mass of the atom

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
Hydrogen exists as molecules with _____ atom(s).
A) seven
B) two
C) four
D) one
E) five
A) seven
B) two
C) four
D) one
E) five

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is name given to SO2 according to chemical nomenclature?
A) Sulfur oxide
B) Oxo-sulfur
C) Sulfur dioxide
D) Sulfo-oxide
E) Dioxy sulfide
A) Sulfur oxide
B) Oxo-sulfur
C) Sulfur dioxide
D) Sulfo-oxide
E) Dioxy sulfide

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following represents the sodium ion?
A) Na2+
B) Na+
C) NaO-
D) Na+OH-
E) Na2-
A) Na2+
B) Na+
C) NaO-
D) Na+OH-
E) Na2-

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
The mass of an electron is _____ atomic mass units.
A) 1.00728
B) 0.000549
C) 0.5490
D) 1.879
E) 1.866
A) 1.00728
B) 0.000549
C) 0.5490
D) 1.879
E) 1.866

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
A chemical bond is a connection between _____.
A) two molecules of a compound
B) electrons and the nucleus of an atom
C) two atoms in a molecule
D) neutrons and protons in the nucleus
E) two or more subatomic particles
A) two molecules of a compound
B) electrons and the nucleus of an atom
C) two atoms in a molecule
D) neutrons and protons in the nucleus
E) two or more subatomic particles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
Electrons move from one atom to another to form species with overall electric charges. Such species are called _____.
A) beams
B) antiparticles
C) ions
D) quarks
E) composite particles
A) beams
B) antiparticles
C) ions
D) quarks
E) composite particles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
How many neutrons can be found in the atom 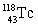 ?
?
A) 43
B) 161
C) 118
D) 75
E) 32
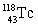 ?
?A) 43
B) 161
C) 118
D) 75
E) 32

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
The molecular mass is the sum of _____ in a molecule.
A) redundant neutrons
B) masses of subatomic particles
C) masses of protons
D) the masses of the atoms
E) electronic masses of atoms
A) redundant neutrons
B) masses of subatomic particles
C) masses of protons
D) the masses of the atoms
E) electronic masses of atoms

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is an example of an anion formed by the gain of two electrons?
A) Br−
B) Cu2+
C) Na+
D) Se2−
E) Rb+ I-
A) Br−
B) Cu2+
C) Na+
D) Se2−
E) Rb+ I-

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
The _____ of an element is a weighted average of the masses of the isotopes that compose an element.
A) atomic number
B) mass number
C) isotopic mass
D) atomic mass
E) nuclear mass
A) atomic number
B) mass number
C) isotopic mass
D) atomic mass
E) nuclear mass

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
Uranium exists as about 98% U-238 and about 2% U-235. What is the atomic mass of uranium?
A) 238.06
B) 237.94
C) 237.14
D) 238.86
E) 239.55
A) 238.06
B) 237.94
C) 237.14
D) 238.86
E) 239.55

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
Substances that exist as two or more atoms connected together so strongly that they behave as a single particle are called _____.
A) compounds
B) polyatoms
C) diatomic compounds
D) subatomic compounds
E) molecules
A) compounds
B) polyatoms
C) diatomic compounds
D) subatomic compounds
E) molecules

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following constitute a proper molecule?
A) Cl
B) H2O
C) N
D) P+
E) O-
A) Cl
B) H2O
C) N
D) P+
E) O-

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
What is the molecular formula of elemental hydrogen?
A) H2
B) H4
C) H5
D) H6
E) H8
A) H2
B) H4
C) H5
D) H6
E) H8

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
Species with overall positive charges are termed _____.
A) quarks
B) beams
C) cations
D) anions
E) antiparticles
A) quarks
B) beams
C) cations
D) anions
E) antiparticles

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following terms represents the species O2-?
A) oxide anion
B) oxide cation
C) oxygen anion
D) oxygen cation
E) oxo-cation
A) oxide anion
B) oxide cation
C) oxygen anion
D) oxygen cation
E) oxo-cation

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
What is the atomic mass of dinitrogen trioxide (atomic mass of oxygen=15.999 u; atomic mass of nitrogen = 14.007 u)?
A) 76.011 u
B) 30. 010u
C) 74.019u
D) 160.012u
E) 60.019u
A) 76.011 u
B) 30. 010u
C) 74.019u
D) 160.012u
E) 60.019u

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is not true in regards to sulfur hexafluoride (SF6)?
A) It has similar physical properties in the vapor phase as sarin
B) It is very to detect
C) It is not a normal part of the atmosphere
D) Used as an aerial tracer for ventilation systems
E) It is toxic and highly reactive
A) It has similar physical properties in the vapor phase as sarin
B) It is very to detect
C) It is not a normal part of the atmosphere
D) Used as an aerial tracer for ventilation systems
E) It is toxic and highly reactive

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
The Cl− ion is formed when a chlorine atom _____.
A) loses one electron
B) gains one electron
C) loses one proton
D) gains one proton
E) gains one proton and loses one electron
A) loses one electron
B) gains one electron
C) loses one proton
D) gains one proton
E) gains one proton and loses one electron

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following represents diphosphorus hexafluoride?
A) (PF3)6
B) P2F4
C) P2FO6
D) P2F6
E) P2O2F6
A) (PF3)6
B) P2F4
C) P2FO6
D) P2F6
E) P2O2F6

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following species is called sodium nitrate?
A) NaNO2
B) Na5N
C) Na3NOH
D) Na2NO
E) NaNO3
A) NaNO2
B) Na5N
C) Na3NOH
D) Na2NO
E) NaNO3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following statements is true for all acids?
A) Acids do not react with metals.
B) Acids do not contain nitrogen.
C) Acids have a sour taste.
D) Acids do not contain oxygen.
E) Acids do not contain water.
A) Acids do not react with metals.
B) Acids do not contain nitrogen.
C) Acids have a sour taste.
D) Acids do not contain oxygen.
E) Acids do not contain water.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
The smallest part of a substance that has the physical and chemical properties of that substance is called a(n) _____.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
The _____ is a subatomic particle with about the same mass as a proton but no charge.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
The connection between two atoms in a molecule is known as a(n) _____.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
_____ is a specific system for naming compounds, in which unique substances get unique names.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
Write the proper ionic formula for the ionic compound obtained from the ions Al3+ and F−.
A) Al3F3
B) (AlF)3
C) AlF
D) Al3F
E) AlF3
A) Al3F3
B) (AlF)3
C) AlF
D) Al3F
E) AlF3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
Polyatomic ions are a group of ions that _____.
A) have same mass number and different atomic numbers
B) contain more than one atom
C) contain more than one positive or negative charge
D) have same atomic number and different mass numbers
E) contain more than one electron
A) have same mass number and different atomic numbers
B) contain more than one atom
C) contain more than one positive or negative charge
D) have same atomic number and different mass numbers
E) contain more than one electron

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
The magnitude of the charge is listed as a right _____ next to the symbol of the element.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following represents acetic acid?
A) HC2H3O2
B) H2C2O4
C) HClO4
D) H3PO4
E) H2SO3
A) HC2H3O2
B) H2C2O4
C) HClO4
D) H3PO4
E) H2SO3

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
An acid is an ionic compound of the _____ dissolved in water.
A) K+ cation
B) H+ cation
C) O- anion
D) Na+ cation
E) OH- anion
A) K+ cation
B) H+ cation
C) O- anion
D) Na+ cation
E) OH- anion

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
Common table salt (sodium chloride), used on foods, is the ionic compound _____.
A) NaCl2
B) SoCl2
C) SoCl
D) NaCl
E) Na2Cl2
A) NaCl2
B) SoCl2
C) SoCl
D) NaCl
E) Na2Cl2

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
The _____ is the sum of the masses of the atoms in a molecule.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following polyatomic ions is an oxyanion?
A) Triiodide
B) Cyanide
C) Ammonium
D) Carbonate
E) Calcium ion
A) Triiodide
B) Cyanide
C) Ammonium
D) Carbonate
E) Calcium ion

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following polyatomic ions is a cation?
A) Bicarbonate
B) Carbonate
C) Chlorate
D) Peroxide
E) Ammonium
A) Bicarbonate
B) Carbonate
C) Chlorate
D) Peroxide
E) Ammonium

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
The _____ of an element is a weighted average of the masses of the isotopes that compose an element.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
Ionic compounds form between:
A) metals and metals.
B) nonmetals and nonmetals
C) semiconductors and carbon alloys
D) metals and nonmetals.
E) semiconductors and nonmetals
A) metals and metals.
B) nonmetals and nonmetals
C) semiconductors and carbon alloys
D) metals and nonmetals.
E) semiconductors and nonmetals

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
_____ is the proper name for the ionic compound formed between the ions K+ and S2−.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
Atoms of the same element with different numbers of neutrons are called _____.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
A species with an overall positive charge is called a(n) _____.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


