Deck 29: Nuclear Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/79
Play
Full screen (f)
Deck 29: Nuclear Physics
1
The alpha radiation first classified by Rutherford was in fact which of the following?
A)helium nuclei
B)high energy quanta
C)electrons
D)positrons
A)helium nuclei
B)high energy quanta
C)electrons
D)positrons
helium nuclei
2
The ratio of the numbers of neutrons to protons in the nucleus of naturally occurring isotopes tends to vary with atomic number in what manner?
A)increases with greater atomic number
B)decreases with greater atomic number
C)is maximum for atomic number = 60
D)remains constant for entire range of atomic numbers
A)increases with greater atomic number
B)decreases with greater atomic number
C)is maximum for atomic number = 60
D)remains constant for entire range of atomic numbers
increases with greater atomic number
3
Rutherford's experiments involving the use of alpha particle beams directed onto thin metal foils demonstrated the existence of which of the following?
A)neutron
B)proton
C)nucleus
D)positron
A)neutron
B)proton
C)nucleus
D)positron
nucleus
4
The experiment, which gave the first evidence for the existence of the atomic nucleus, involved which of the following?
A)x-ray scattering
B)radioactive dating
C)cosmic ray detection
D)alpha scattering
A)x-ray scattering
B)radioactive dating
C)cosmic ray detection
D)alpha scattering

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
5
The mass of 12C is 12 u where 1 u = 1.660 559 * 10-27 kg.This mass is equal to:
A)the mass of the 12C nucleus.
B)the mass of the 12C nucleus plus 6 electrons.
C)the mass of the 12C nucleus plus 12 electrons.
D)the mass of 6 protons and 6 neutrons.
A)the mass of the 12C nucleus.
B)the mass of the 12C nucleus plus 6 electrons.
C)the mass of the 12C nucleus plus 12 electrons.
D)the mass of 6 protons and 6 neutrons.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
6
The atomic number of a given element is equivalent to which of the following?
A)proton number in the nucleus
B)neutron number in the nucleus
C)sum of the protons and neutrons in the nucleus
D)number of electrons in the outer shells
A)proton number in the nucleus
B)neutron number in the nucleus
C)sum of the protons and neutrons in the nucleus
D)number of electrons in the outer shells

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
7
If there are 146 neutrons in 238U, how many neutrons are found in the nucleus of 235U?
A)141
B)143
C)145
D)147
A)141
B)143
C)145
D)147

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
8
The gamma radiation first classified by Rutherford was in fact which of the following?
A)helium nuclei
B)high energy quanta
C)electrons
D)positrons
A)helium nuclei
B)high energy quanta
C)electrons
D)positrons

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
9
Neglecting recoil of the gold nucleus, how much kinetic energy must an alpha particle (charge = 2 * 1.6 * 10-19 C) have to approach to within 1.00 * 10-14 m of a gold nucleus (charge = 79 * 1.6 * 10-19 C)? (ke = 8.99 * 109 N*m2/C2 and 1 MeV = 1.6 * 10-13 J)
A)11.7 MeV
B)14.6 MeV
C)18.2 MeV
D)22.7 MeV
A)11.7 MeV
B)14.6 MeV
C)18.2 MeV
D)22.7 MeV

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
10
The binding energy of a nucleus is equal to:
A)the energy needed to remove one of the nucleons.
B)the average energy with which any nucleon is bound in the nucleus.
C)the energy needed to separate all the nucleons from each other.
D)the mass of the nucleus times c2.
A)the energy needed to remove one of the nucleons.
B)the average energy with which any nucleon is bound in the nucleus.
C)the energy needed to separate all the nucleons from each other.
D)the mass of the nucleus times c2.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
11
The isotope 64Zn has a nuclear radius of 4.8 * 10-15 m.Approximately what is the nuclear radius of the isotope 27Al?
A)2.0 * 10-15 m
B)2.7 * 10-15 m
C)3.6 * 10-15 m
D)4.0 * 10-15 m
A)2.0 * 10-15 m
B)2.7 * 10-15 m
C)3.6 * 10-15 m
D)4.0 * 10-15 m

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
12
The atomic mass number of a nucleus is equivalent to which of the following numbers?
A)number of neutrons present
B)number of protons present
C)difference in neutron and proton numbers
D)sum of neutron and proton numbers
A)number of neutrons present
B)number of protons present
C)difference in neutron and proton numbers
D)sum of neutron and proton numbers

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
13
What is the binding energy per nucleon of 197Au (atomic number = 79)? (The following information regarding atomic masses will be needed: 197Au, 196.966 543 u; hydrogen, 1.007 825 u; neutron, 1.008 665 u; also 1 u = 931.5 MeV/c2)
A)7.3 MeV
B)7.7 MeV
C)7.9 MeV
D)8.3 MeV
A)7.3 MeV
B)7.7 MeV
C)7.9 MeV
D)8.3 MeV

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
14
The isotope 64Zn has a nuclear radius of 4.8 *10-15 m.Which of the following is the mass number of an isotope for which the nuclear radius is 7.2 *10-15 m?
A)144
B)96
C)125
D)216
A)144
B)96
C)125
D)216

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
15
What energy must be added or given off in a reaction where two hydrogen atoms and two neutrons are combined to form a helium atom? (Atomic masses for each: hydrogen, 1.007 825 u; neutron, 1.008 665 u; helium, 4.002 602 u; also, 1 u = 931.5 MeV/c2)
A)20.7 MeV added
B)20.7 MeV given off
C)28.3 MeV given off
D)28.3 MeV added
A)20.7 MeV added
B)20.7 MeV given off
C)28.3 MeV given off
D)28.3 MeV added

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
16
For every stable nucleus except hydrogen, if the number of nucleons is doubled, the quantity that will change least is the:
A)mass.
B)charge.
C)volume.
D)density.
A)mass.
B)charge.
C)volume.
D)density.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
17
The molecular mass for chlorine is not an integer multiple of the molecular mass of hydrogen but is about 35.5 times the molecular mass of hydrogen.This is primarily because:
A)the proton and neutron have different masses.
B)there are different isotopes of chlorine.
C)of the binding energy of the chlorine nucleus.
D)the chlorine nucleus has 35.5 nucleons.
A)the proton and neutron have different masses.
B)there are different isotopes of chlorine.
C)of the binding energy of the chlorine nucleus.
D)the chlorine nucleus has 35.5 nucleons.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
18
The nucleus of an atom is made up of which of the following?
A)electrons and protons
B)electrons and neutrons
C)protons, electrons and neutrons
D)protons and neutrons
A)electrons and protons
B)electrons and neutrons
C)protons, electrons and neutrons
D)protons and neutrons

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
19
The beta radiation first classified by Rutherford was in fact which of the following?
A)helium nuclei
B)high energy quanta
C)electrons
D)positrons
A)helium nuclei
B)high energy quanta
C)electrons
D)positrons

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
20
Certain stars at the end of their lives are thought to collapse combining their protons and electrons together to form a neutron star.Such a star could be thought of as a giant atomic nucleus.If a star of mass equal to that of the sun (M = 1.99 * 1030 kg) collapsed into neutrons (mn = 1.67 * 10-27 kg), what would be the radius of such a star? (Hint: r = r0A1/3, where r0 = 1.20 * 10-15 m).
A)25.4 km
B)18.7 km
C)12.7 km
D)6.40 km
A)25.4 km
B)18.7 km
C)12.7 km
D)6.40 km

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
21
Tritium is radioactive with a half-life of 12.33 years decaying into 3He with low-energy electron emission.If we have a sample of 3.00 * 1018 tritium atoms, what is its activity in decays/second? (1 year = 3.15 * 107 s)
A)4.20 * 1010/second
B)5.35 * 109 /second
C)3.69 * 108/second
D)6.64 * 107/second
A)4.20 * 1010/second
B)5.35 * 109 /second
C)3.69 * 108/second
D)6.64 * 107/second

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
22
There are samples of two different isotopes, X and Y.Both contain the same number of radioactive atoms.Sample X has a half-life twice that of Y.How do their decay rates compare?
A)X has a greater rate than Y.
B)X has a smaller rate than Y.
C)The rates of X and Y are equal.
D)The rate depends on atomic number, not half-life.
A)X has a greater rate than Y.
B)X has a smaller rate than Y.
C)The rates of X and Y are equal.
D)The rate depends on atomic number, not half-life.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
23
The half-life of 18N is 0.62 s. What is the decay constant for this isotope?
A)0.43 s-1
B)1.1 s-1
C)1.7 * 10-11 Ci
D)The decay constant is not defined for a half-life of less than one second.
A)0.43 s-1
B)1.1 s-1
C)1.7 * 10-11 Ci
D)The decay constant is not defined for a half-life of less than one second.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
24
A pure sample of 226Ra contains 2.0 * 1014 atoms of the isotope.If the half-life of 226Ra = 1.6 * 103 years, what is the decay rate of this sample? (1 Ci = 3.7 * 1010 decays/s)
A)2.7 * 10-12 Ci
B)3.4 * 10-10 Ci
C)7.4 * 10-8 Ci
D)9.6 * 10-6 Ci
A)2.7 * 10-12 Ci
B)3.4 * 10-10 Ci
C)7.4 * 10-8 Ci
D)9.6 * 10-6 Ci

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
25
A radioactive material initially is observed to have an activity of 1 000 decays/sec.If three hours later it is observed to have an activity of 125 decays/sec, what is its half-life?
A)1/2 hour
B)1 hour
C)3 hours
D)8 hours
A)1/2 hour
B)1 hour
C)3 hours
D)8 hours

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
26
Approximately how many half-life periods must elapse if the activity of a radioactive isotope sample is to be reduced to 0.004 of the original value?
A)3
B)6
C)8
D)60
A)3
B)6
C)8
D)60

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
27
If the stable nuclei are plotted with neutron number vs.proton number, the curve formed by the stable nuclei does not follow the line N = Z.This is predicted by examining how the binding energy is influenced by:
A)the volume of the nucleus.
B)the size of the nuclear surface.
C)the Coulomb repulsion.
D)the proton-neutron mass difference.
A)the volume of the nucleus.
B)the size of the nuclear surface.
C)the Coulomb repulsion.
D)the proton-neutron mass difference.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
28
The half-life of radioactive Technetium-99 is 6.0 hours.Find the number of 99Tc nuclei necessary to produce a sample of activity 1.0 µCi.(1 Ci = 3.7 * 1010 decays/second)
A)8.0 * 108
B)1.2 * 109
C)2.1 * 1010
D)3.4 * 1011
A)8.0 * 108
B)1.2 * 109
C)2.1 * 1010
D)3.4 * 1011

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
29
The fact that the binding energy per nucleon does not depend very strongly on the volume of the nucleus indicates that:
A)the strong nuclear force saturates.
B)nucleons don't move throughout the nucleus.
C)all nuclei have the same volume.
D)the radius of a nucleus is directly proportional to the number of nucleons.
A)the strong nuclear force saturates.
B)nucleons don't move throughout the nucleus.
C)all nuclei have the same volume.
D)the radius of a nucleus is directly proportional to the number of nucleons.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
30
A pure sample of 226Ra contains 2.0 * 1014 atoms of the isotope.If the half-life of 226Ra = 1.6 * 103 years, what is the activity of this sample?
A)6.7 * 109 decays/yr
B)8.7 * 1010 decays/yr
C)9.4 * 1010 decays/yr
D)13 * 1010 decays/yr
A)6.7 * 109 decays/yr
B)8.7 * 1010 decays/yr
C)9.4 * 1010 decays/yr
D)13 * 1010 decays/yr

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
31
Tritium has a half-life of 12.3 years.How many years will elapse when the radioactivity of a tritium sample diminishes to 20% of its original value?
A)21 years
B)29 years
C)57 years
D)86 years
A)21 years
B)29 years
C)57 years
D)86 years

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
32
An element is emitting alpha, beta, and gamma radiation.Rank order them in terms of the thickness of protective shielding they will need for safety, from least to most.
A)alpha, beta and gamma
B)gamma, beta and alpha
C)beta, gamma and alpha
D)alpha, gamma and beta
A)alpha, beta and gamma
B)gamma, beta and alpha
C)beta, gamma and alpha
D)alpha, gamma and beta

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the binding energy per nucleon of the tritium nucleus, 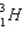 , given that the mass of the tritium nucleus is 3.016 05 u.(mp = 1.007 276 u, mn = 1.008 665, and 1 u = 931.5 MeV/c2)
, given that the mass of the tritium nucleus is 3.016 05 u.(mp = 1.007 276 u, mn = 1.008 665, and 1 u = 931.5 MeV/c2)
A)2.24 MeV/nucleon
B)2.45 MeV/nucleon
C)2.66 MeV/nucleon
D)2.86 MeV/nucleon
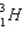 , given that the mass of the tritium nucleus is 3.016 05 u.(mp = 1.007 276 u, mn = 1.008 665, and 1 u = 931.5 MeV/c2)
, given that the mass of the tritium nucleus is 3.016 05 u.(mp = 1.007 276 u, mn = 1.008 665, and 1 u = 931.5 MeV/c2)A)2.24 MeV/nucleon
B)2.45 MeV/nucleon
C)2.66 MeV/nucleon
D)2.86 MeV/nucleon

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
34
1 Bq = ________ Ci?
A)1
B)106
C)2.7 * 10-11
D)3.7 * 1010
A)1
B)106
C)2.7 * 10-11
D)3.7 * 1010

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
35
The mass of 238U is not quite an integer multiple of 1H mass.This is primarily because:
A)the proton and neutron have different masses.
B)there are several isotopes of uranium.
C)of the binding energy of uranium.
D)uranium is radioactive.
A)the proton and neutron have different masses.
B)there are several isotopes of uranium.
C)of the binding energy of uranium.
D)uranium is radioactive.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
36
An ancient building was known to have been built 3 000 years ago.Approximately what proportion of Carbon-14 atoms are yet in the building's wooden framing compared to the number which were present at the time of its construction? (half life of 14C = 5 730 years)
A)0.425
B)0.500
C)0.517
D)0.696
A)0.425
B)0.500
C)0.517
D)0.696

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
37
If a fossil bone is found to contain 1/8th as much Carbon-14 as the bone of a living animal, what is the approximate age of the fossil? (half-life of 14C = 5 730 years)
A)7 640 years
B)17 200 years
C)22 900 years
D)45 800 years
A)7 640 years
B)17 200 years
C)22 900 years
D)45 800 years

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
38
Tritium has a half-life of 12.3 years.What proportion of its original radioactivity will a sample have after 9 years?
A)0.55
B)0.60
C)0.73
D)0.84
A)0.55
B)0.60
C)0.73
D)0.84

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
39
Approximately how many radioactive atoms are present in a tritium sample with an activity of 0.4 * 10-6 Ci and a half-life of 12.3 years? (1 Ci = 3.7 * 1010 decays/s)
A)1.3 * 108
B)7 * 108
C)3 * 1010
D)8 * 1012
A)1.3 * 108
B)7 * 108
C)3 * 1010
D)8 * 1012

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
40
Over the course of 3 hours, 15% of a radioactive material decays.What is its half-life?
A)4.1 hrs
B)12.8 hrs
C)24.0 hrs
D)68.6 hrs
A)4.1 hrs
B)12.8 hrs
C)24.0 hrs
D)68.6 hrs

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
41
Of the main types of radiation emitted from naturally radioactive isotopes, which of the following is the most penetrating?
A)alpha
B)beta (electron)
C)gamma
D)beta (positron)
A)alpha
B)beta (electron)
C)gamma
D)beta (positron)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
42
A radioactive isotope that emits a gamma quantum will change in what respect?
A)Atomic number increases by one.
B)Atomic number decreases by one.
C)Atomic mass number decreases by one.
D)None of the above choices are valid.
A)Atomic number increases by one.
B)Atomic number decreases by one.
C)Atomic mass number decreases by one.
D)None of the above choices are valid.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
43
The existence of the neutrino was postulated to account for which basic conservation laws during the beta decay process?
A)conservation of energy
B)conservation of momentum
C)Both choices a and b are valid.
D)None of the above choices are valid.
A)conservation of energy
B)conservation of momentum
C)Both choices a and b are valid.
D)None of the above choices are valid.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
44
Chromium-55 (54.940 8 u) emits an electron leaving a daughter nucleus of manganese-55 (54.938 0 u).How much energy is released in this reaction? (1 u = 931.5 MeV/c2)
A)5.59 MeV
B)2.61 MeV
C)1.40 MeV
D)0.70 MeV
A)5.59 MeV
B)2.61 MeV
C)1.40 MeV
D)0.70 MeV

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
45
The neutrino is:
A)a little neutron.
B)another name for a positron.
C)the particle detected in carbon dating.
D)a particle proposed to account for "missing" energy and momentum in beta decay.
A)a little neutron.
B)another name for a positron.
C)the particle detected in carbon dating.
D)a particle proposed to account for "missing" energy and momentum in beta decay.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
46
An alpha particle (mass = 6.68 * 10-27 kg) is emitted from a radioactive nucleus with an energy of 5.00 MeV.How fast is the alpha particle moving in m/s? (1 MeV = 1.6 * 10-13 J)
A)2.40 * 107 m/s
B)1.55 * 107 m/s
C)3.70 * 106 m/s
D)1.85 * 106 m/s
A)2.40 * 107 m/s
B)1.55 * 107 m/s
C)3.70 * 106 m/s
D)1.85 * 106 m/s

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
47
What particle is emitted when 20Na decays to 20Ne? (atomic numbers of Na and Ne are, respectively, 11 and 10)
A)alpha
B)beta (electron)
C)beta (positron)
D)gamma quantum
A)alpha
B)beta (electron)
C)beta (positron)
D)gamma quantum

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
48
Tritium (3H) has a half-life of 12.3 years and releases 0.018 6 MeV energy per decay. What is the rate at which energy is released for a 1.0-gram sample of tritium? (NA = 6.02 * 1023 mol-1, 1 year = 3.16 * 107 s, 1 MeV = 1.6 * 10-13 J, and mt = 3.016 05 u)
A)1.1 W
B)9.6 W
C)3.2 W
D)0.33 W
A)1.1 W
B)9.6 W
C)3.2 W
D)0.33 W

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
49
In the beta decay of 14C, the existence of the antineutrino was required to maintain:
A)energy conservation.
B)charge conservation.
C)conservation of the number of nucleons.
D)all of the above.
A)energy conservation.
B)charge conservation.
C)conservation of the number of nucleons.
D)all of the above.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
50
The alpha emission process results in the daughter nucleus differing in what manner from the parent?
A)Atomic mass increases by one.
B)Atomic number decreases by two.
C)Atomic number increases by one.
D)Atomic mass decreases by two.
A)Atomic mass increases by one.
B)Atomic number decreases by two.
C)Atomic number increases by one.
D)Atomic mass decreases by two.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
51
Each of the three naturally occurring radioactive series start with one of the following isotopes except for which one?
A)(238U)
B)(235U)
C)(232Th)
D)(237Np)
A)(238U)
B)(235U)
C)(232Th)
D)(237Np)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
52
The neutron is radioactive and can beta decay to form a proton.This can occur primarily because:
A)the proton has less mass than a neutron.
B)there are several hydrogen isotopes.
C)of the binding energy of hydrogen.
D)the neutron is neutral.
A)the proton has less mass than a neutron.
B)there are several hydrogen isotopes.
C)of the binding energy of hydrogen.
D)the neutron is neutral.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
53
When radium-224 emits an alpha particle, the remaining daughter nucleus is which of the following?
A)lead-213
B)actinium-215
C)radon-220
D)bismuth-215
A)lead-213
B)actinium-215
C)radon-220
D)bismuth-215

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
54
Tritium (3H) has a half-life of 12.3 years and releases 0.018 6 MeV energy per decay. What is the activity of a 1.0-gram sample of tritium? (NA = 6.02 * 1023 mol-1, 1 year = 3.16 * 107 s, 1 MeV = 1.6 * 10-13 J, and mt = 3.016 05 u)
A)1.1 * 1015 Bq
B)3.2 * 1015 Bq
C)3.6 * 1014 Bq
D)1.3 * 1016 Bq
A)1.1 * 1015 Bq
B)3.2 * 1015 Bq
C)3.6 * 1014 Bq
D)1.3 * 1016 Bq

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
55
The original nucleus and the final nucleus will be different isotopes of the same element for which decay scheme of the original nucleus?
A)alpha decay followed by two beta (electron) decays
B)two gamma decays
C)a beta (electron) decay followed by an alpha decay
D)a beta (electron) decay followed by neutron emission
A)alpha decay followed by two beta (electron) decays
B)two gamma decays
C)a beta (electron) decay followed by an alpha decay
D)a beta (electron) decay followed by neutron emission

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
56
The beta emission process results in the daughter nucleus differing in what manner from the parent?
A)Atomic mass changes by one.
B)Atomic number changes by two.
C)Atomic number changes by one.
D)Atomic mass changes by two.
A)Atomic mass changes by one.
B)Atomic number changes by two.
C)Atomic number changes by one.
D)Atomic mass changes by two.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
57
What particle is emitted when 240Pu decays to 236U? (atomic numbers of Pu and U are, respectively, 94 and 92)
A)alpha
B)beta (electron)
C)beta (positron)
D)gamma quantum
A)alpha
B)beta (electron)
C)beta (positron)
D)gamma quantum

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
58
A radioactive isotope that emits an alpha particle will change in what respect?
A)Atomic number decreases by four.
B)Mass number decreases by four.
C)Both choices a and b are valid.
D)None of the above choices are valid.
A)Atomic number decreases by four.
B)Mass number decreases by four.
C)Both choices a and b are valid.
D)None of the above choices are valid.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
59
Uranium-238 decays to Thorium-234 by emitting which of the following?
A)beta
B)alpha
C)gamma
D)positron
A)beta
B)alpha
C)gamma
D)positron

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
60
A 1-gram sample of wood is taken from an ancient site.If the Carbon-14 activity of the sample is 12.5% that of present-day organic material, what is the age of the wood? (T1/2 for 14C is 5 730 years)
A)4 460 years
B)8 600 years
C)13 150 years
D)17 200 years
A)4 460 years
B)8 600 years
C)13 150 years
D)17 200 years

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
61
What is the Q value of the reaction 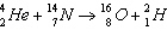 ? The mass of the alpha particle is 4.002 602 u, of the nitrogen is 14.003 074 u, of the oxygen is 15.994 915 u, and of the hydrogen is 2.014 102 u.(1 u = 931.5 MeV/c2)
? The mass of the alpha particle is 4.002 602 u, of the nitrogen is 14.003 074 u, of the oxygen is 15.994 915 u, and of the hydrogen is 2.014 102 u.(1 u = 931.5 MeV/c2)
A)-3.34 MeV
B)3.34 MeV
C)-3.11 MeV
D)3.11 MeV
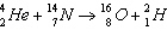 ? The mass of the alpha particle is 4.002 602 u, of the nitrogen is 14.003 074 u, of the oxygen is 15.994 915 u, and of the hydrogen is 2.014 102 u.(1 u = 931.5 MeV/c2)
? The mass of the alpha particle is 4.002 602 u, of the nitrogen is 14.003 074 u, of the oxygen is 15.994 915 u, and of the hydrogen is 2.014 102 u.(1 u = 931.5 MeV/c2)A)-3.34 MeV
B)3.34 MeV
C)-3.11 MeV
D)3.11 MeV

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
62
In the four radioactive series, the nuclei decay by either emitting alpha particles or beta particles until they reach the stable end product.Each decay, therefore, results in a mass number change of either 4 (for alpha decay) or 0 (for beta decay).The radium isotope 226Ra is in one of these series.What is the starting isotope in the series containing 226Ra?
A)(238U)
B)(235U)
C)(232Th)
D)(237Np)
A)(238U)
B)(235U)
C)(232Th)
D)(237Np)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
63
Two nuclei collide, one originally at rest, in an endothermic nuclear reaction with Q = -2.05 MeV.Which of the following describes the minimum kinetic energy needed in the reactant nuclei for the reaction is to occur?
A)equal to 2.05 MeV
B)greater than 2.05 MeV
C)less than 2.05 MeV
D)exactly half of 2.05 MeV
A)equal to 2.05 MeV
B)greater than 2.05 MeV
C)less than 2.05 MeV
D)exactly half of 2.05 MeV

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
64
The Q of a nuclear reaction is equal to:
A)the total charge involved.
B)energy associated with the change in mass.
C)energy associated with momentum conservation.
D)the exothermic endothermy.
A)the total charge involved.
B)energy associated with the change in mass.
C)energy associated with momentum conservation.
D)the exothermic endothermy.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
65
The Q value of the reaction 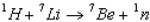 is -1.65 MeV.What is the threshold energy of this reaction? (Hint: Once the Q value is known, the relative mass numbers are precise enough to yield the result to 3 significant figures, actual masses not being needed).
is -1.65 MeV.What is the threshold energy of this reaction? (Hint: Once the Q value is known, the relative mass numbers are precise enough to yield the result to 3 significant figures, actual masses not being needed).
A)1.65 MeV
B)1.89 MeV
C)1.77 MeV
D)1.41 MeV
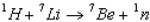 is -1.65 MeV.What is the threshold energy of this reaction? (Hint: Once the Q value is known, the relative mass numbers are precise enough to yield the result to 3 significant figures, actual masses not being needed).
is -1.65 MeV.What is the threshold energy of this reaction? (Hint: Once the Q value is known, the relative mass numbers are precise enough to yield the result to 3 significant figures, actual masses not being needed).A)1.65 MeV
B)1.89 MeV
C)1.77 MeV
D)1.41 MeV

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is not a unit of radiation dose?
A)rem
B)roentgen
C)rad
D)RBE
A)rem
B)roentgen
C)rad
D)RBE

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
67
Two different nuclei emit alpha particles, the energy released in each of these decays being the same.Which of the following has the highest resulting kinetic energy?
A)The lighter daughter nucleus.
B)The heavier daughter nucleus.
C)The alpha particle from the lighter nucleus.
D)The alpha particle from the heavier nucleus.
A)The lighter daughter nucleus.
B)The heavier daughter nucleus.
C)The alpha particle from the lighter nucleus.
D)The alpha particle from the heavier nucleus.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
68
What is the Q-value for the reaction where the products are 0.005 0 u less than the reactants? (1 u = 931.5 MeV/c2)
A)8.5 MeV
B)7.6 MeV
C)5.2 MeV
D)4.7 MeV
A)8.5 MeV
B)7.6 MeV
C)5.2 MeV
D)4.7 MeV

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
69
If the sum total mass of reactants in a nuclear reaction is greater than that of product particles, then which of the following statements best describes the conditions of the reaction?
A)reaction is exothermic
B)reaction is endothermic
C)atomic number of each reactant must be greater than 40
D)atomic number of each reactant must be less than 80
A)reaction is exothermic
B)reaction is endothermic
C)atomic number of each reactant must be greater than 40
D)atomic number of each reactant must be less than 80

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
70
The isotope 238U, which starts one of the natural radioactive series, decays first by alpha decay followed by two negative beta decays.At this point, what is the resulting isotope?
A)(238U)
B)(236U)
C)(234Th (Z = 90 for Th.))
D)Some other uranium isotope not given above.
A)(238U)
B)(236U)
C)(234Th (Z = 90 for Th.))
D)Some other uranium isotope not given above.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following is not true for both the photon and the neutrino.
A)Both are uncharged.
B)Both have spin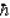 .
.
C)Both can carry different amounts of momentum.
D)Choose this answer if all of the above are true.
A)Both are uncharged.
B)Both have spin
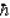 .
.C)Both can carry different amounts of momentum.
D)Choose this answer if all of the above are true.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
72
A particle is fired at a target nucleus in which a reaction that could occur has a negative Q value.Which of the following statements is true?
A)The kinetic energy of the bombarding particle can be any amount for the reaction to occur.
B)The kinetic energy of the bombarding particle must be equal to the absolute value of the Q value for the reaction to occur.
C)The kinetic energy of the bombarding particle was greater than the absolute value of the Q value if the reaction occurred.
D)The Q value has nothing to do with whether or not the reaction can occur.
A)The kinetic energy of the bombarding particle can be any amount for the reaction to occur.
B)The kinetic energy of the bombarding particle must be equal to the absolute value of the Q value for the reaction to occur.
C)The kinetic energy of the bombarding particle was greater than the absolute value of the Q value if the reaction occurred.
D)The Q value has nothing to do with whether or not the reaction can occur.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
Genetic radiation damage is:
A)another name for somatic damage.
B)any radiation damage to a cell.
C)radiation damage affecting reproductive cells.
D)measured in roentgens.
A)another name for somatic damage.
B)any radiation damage to a cell.
C)radiation damage affecting reproductive cells.
D)measured in roentgens.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
A proton is captured by an oxygen-16 atom which in turn emits a deuteron.What is the element and mass number of the product isotope?
A)nitrogen-15
B)oxygen-17
C)oxygen-15
D)fluorine-15
A)nitrogen-15
B)oxygen-17
C)oxygen-15
D)fluorine-15

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
In the reaction 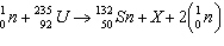 , what is the mass number and atomic number of the product designated by X?
, what is the mass number and atomic number of the product designated by X?
A)102, 42
B)101, 42
C)102, 44
D)not given
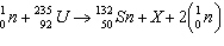 , what is the mass number and atomic number of the product designated by X?
, what is the mass number and atomic number of the product designated by X?A)102, 42
B)101, 42
C)102, 44
D)not given

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
76
Sample #1 is made from an isotope with decay constant 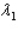 and sample #2 is made from an isotope with decay constant
and sample #2 is made from an isotope with decay constant 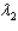 , where
, where 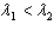 .Which of the following statements must be true?
.Which of the following statements must be true?
A)The activity of sample #1 is greater than that of sample #2.
B)The activity of sample #2 is greater than that of sample #1.
C)The half-life exhibited for sample #1 is greater than that of sample #2.
D)The half-life exhibited for sample #2 is greater than that for sample #1
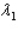 and sample #2 is made from an isotope with decay constant
and sample #2 is made from an isotope with decay constant 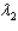 , where
, where 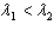 .Which of the following statements must be true?
.Which of the following statements must be true?A)The activity of sample #1 is greater than that of sample #2.
B)The activity of sample #2 is greater than that of sample #1.
C)The half-life exhibited for sample #1 is greater than that of sample #2.
D)The half-life exhibited for sample #2 is greater than that for sample #1

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
77
A rad is that amount of radiation that:
A)produces 2.08 * 109 ion pairs per cm3 in air under standard conditions.
B)deposits 8.76 * 10-3 J of energy into 1 kg of air.
C)deposits 10-2 J of energy into 1 kg of absorbing material.
D)is also known as a rem.
A)produces 2.08 * 109 ion pairs per cm3 in air under standard conditions.
B)deposits 8.76 * 10-3 J of energy into 1 kg of air.
C)deposits 10-2 J of energy into 1 kg of absorbing material.
D)is also known as a rem.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
78
The isotope 14C cannot be used in dating old samples of which of the following?
A)charcoal from a fire
B)a bronze implement from a cave
C)a bone buried in mud
D)All of the above can be dated using 14C.
A)charcoal from a fire
B)a bronze implement from a cave
C)a bone buried in mud
D)All of the above can be dated using 14C.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
79
To what is the radiation damage in biological organisms primarily due?
A)helium introduction
B)heating
C)induced radioactivity
D)ionization
A)helium introduction
B)heating
C)induced radioactivity
D)ionization

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck


