Deck 12: Energy and Hydrocarbons
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/46
Play
Full screen (f)
Deck 12: Energy and Hydrocarbons
1
Which is a characteristic feature of alkenes?
A) general formula of CnH2n + 2
B) tetrahedral geometry
C) unsaturated
D) hydrocarbons which contain only single bonds
A) general formula of CnH2n + 2
B) tetrahedral geometry
C) unsaturated
D) hydrocarbons which contain only single bonds
unsaturated
2
All combustion reactions of fossil fuels
A) give off energy
B) are complete combustions with oxygen
C) are endothermic reactions
D) are l00% efficient
A) give off energy
B) are complete combustions with oxygen
C) are endothermic reactions
D) are l00% efficient
give off energy
3
Hydrocarbons with triple bonds are called
A) alkanes
B) alkenes
C) alkynes
D) aromatic hydrocarbons
A) alkanes
B) alkenes
C) alkynes
D) aromatic hydrocarbons
alkynes
4
Which of the following will not improve the octane rating of a gasoline?
A) increasing the percentage of branched-chain hydrocarbons
B) increasing the percentage of aromatic hydrocarbons
C) increasing the percentage of straight-chain hydrocarbons
D) adding octane enhancers
A) increasing the percentage of branched-chain hydrocarbons
B) increasing the percentage of aromatic hydrocarbons
C) increasing the percentage of straight-chain hydrocarbons
D) adding octane enhancers

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
5
The symbol 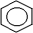 represents
represents
A) a cycloalkane
B) cyclohexane
C) hexane
D) benzene
 represents
representsA) a cycloalkane
B) cyclohexane
C) hexane
D) benzene

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
6
The name of the C2H5- group is
A) methyl
B) ethyl
C) propyl
D) butyl
A) methyl
B) ethyl
C) propyl
D) butyl

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
7
Which hydrocarbon is the principal component of natural gas?
A) methane
B) benzene
C) ethylene
D) isooctane
A) methane
B) benzene
C) ethylene
D) isooctane

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is ethylene?
A) CH2::CHCH3
B) CH2::CH2
C) H-C:::C-H
D) CH3-CH3
A) CH2::CHCH3
B) CH2::CH2
C) H-C:::C-H
D) CH3-CH3

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
9
Fermentation of carbohydrates is a source of
A) MTBE
B) ethanol
C) ethers
D) methanol
A) MTBE
B) ethanol
C) ethers
D) methanol

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
10
At current usage rates, world reserves of which fuel below are estimated to last for the least number of years?
A) natural gas
B) hydrogen
C) petroleum
D) coal
A) natural gas
B) hydrogen
C) petroleum
D) coal

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
11
Which fossil fuel furnishes the most heat energy per gram?
A) petroleum
B) charcoal
C) natural gas
D) coal
A) petroleum
B) charcoal
C) natural gas
D) coal

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
12
Which is a characteristic of alkanes?
A) contain C=C
B) general formula CnH2n
C) saturated
D) some isomers are cis and trans
A) contain C=C
B) general formula CnH2n
C) saturated
D) some isomers are cis and trans

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
13
The complete combustion of C2H6 yields
A) C2H4 and H2
B) CO and H2O
C) CO2 and H2O
D) C and H2
A) C2H4 and H2
B) CO and H2O
C) CO2 and H2O
D) C and H2

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is an isomer of 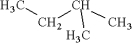
A)
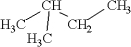
B)
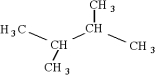
C)
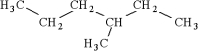
D)
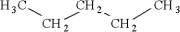
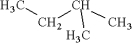
A)
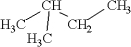
B)

C)

D)
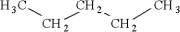

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
15
Which is a representation of cyclohexane?
A)
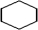
B) C6H6
C)
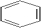
D)
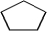
A)

B) C6H6
C)

D)


Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
16
Butane [CH3CH2CH2CH3] and 2-methylpropane [(CH3)2CHCH3] are
A) stereoisomers
B) cis-trans isomers
C) structural isomers
D) optical isomers
A) stereoisomers
B) cis-trans isomers
C) structural isomers
D) optical isomers

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
17
Coal gasification is a process for making
A) synthesis gas
B) molecules that are precursors of organic chemicals
C) methane
D) all of these
A) synthesis gas
B) molecules that are precursors of organic chemicals
C) methane
D) all of these

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
18
Which process is used to increase the amount of the gasoline fraction in refining to match commercial demand?
A) reforming
B) oxygenating
C) cracking
D) gasification
A) reforming
B) oxygenating
C) cracking
D) gasification

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
19
What is the bond angle around a triple bond?
A) 90
B) l09.5
C) l20
D) l80
A) 90
B) l09.5
C) l20
D) l80

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
20
A compound containing seven carbon atoms is
A) heptane
B) octane
C) hexane
D) nonane
A) heptane
B) octane
C) hexane
D) nonane

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
21
Gasohol contains
A) up to 10% ethanol mixed with unleaded gasoline
B) up to 10% methanol mixed with unleaded gasoline
C) three grams of tetraethyl lead
D) three grams of tertiary-butyl alcohol per gallon
A) up to 10% ethanol mixed with unleaded gasoline
B) up to 10% methanol mixed with unleaded gasoline
C) three grams of tetraethyl lead
D) three grams of tertiary-butyl alcohol per gallon

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is not true about benzene?
A) has the formula C6H12
B) is a carcinogen
C) increases the octane rating of gasoline
D) is a cyclic hydrocarbon
A) has the formula C6H12
B) is a carcinogen
C) increases the octane rating of gasoline
D) is a cyclic hydrocarbon

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
23
Which is a characteristic of alkanes?
A) contain carbon carbon triple bonds C:::C
B) general formula CnH2n-2
C) have only single bonds
D) have isomers are cis and trans
A) contain carbon carbon triple bonds C:::C
B) general formula CnH2n-2
C) have only single bonds
D) have isomers are cis and trans

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following compounds would you expect to have the highest octane rating?
A) pentane, C5H12 , CH3CH2CH2CH2CH3
B) hexane, C6H14 , CH3CH2CH2CH2CH2CH3
C) isooctane, C8H18 , (CH3) 3CCH2CH(CH3) 2
D) heptane, C7H16 , CH3CH2CH2CH2CH2CH2CH3
A) pentane, C5H12 , CH3CH2CH2CH2CH3
B) hexane, C6H14 , CH3CH2CH2CH2CH2CH3
C) isooctane, C8H18 , (CH3) 3CCH2CH(CH3) 2
D) heptane, C7H16 , CH3CH2CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
25
Fractional distillation of petroleum
A) separates and purifies all of the hydrocarbons in crude oil
B) separates fractions, each of which contain many hydrocarbons
C) is the final step in refining gasoline
D) fractions large molecules into smaller ones
A) separates and purifies all of the hydrocarbons in crude oil
B) separates fractions, each of which contain many hydrocarbons
C) is the final step in refining gasoline
D) fractions large molecules into smaller ones

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
26
Which fossil fuel has a molecular structure containing cyclic hydrocarbons?
A) coal
B) liquid propane
C) natural gas
D) none of these
A) coal
B) liquid propane
C) natural gas
D) none of these

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
27
The fuel identified as M85 is
A) a substance with a molar mass of 85
B) a fuel mixture of 85% methanol and 15% gasoline
C) a fuel with a boiling point aboe 85 C
D) a fuel tha is 85% gasoline and 15% ethanol
A) a substance with a molar mass of 85
B) a fuel mixture of 85% methanol and 15% gasoline
C) a fuel with a boiling point aboe 85 C
D) a fuel tha is 85% gasoline and 15% ethanol

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
28
Which alcohol is called wood alcohol?
A) ethanol
B) isopropyl alcohol
C) 1-propanol
D) methanol
A) ethanol
B) isopropyl alcohol
C) 1-propanol
D) methanol

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
29
Which fossil fuel burns cleaner and more completely?
A) coal
B) natural gas
C) petroleum
D) wood
A) coal
B) natural gas
C) petroleum
D) wood

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
30
Carcinogens are compounds that are
A) never used as fuels
B) cancer causing agents
C) not found in petroleum
D) rarely aromatic
A) never used as fuels
B) cancer causing agents
C) not found in petroleum
D) rarely aromatic

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
31
A coal gasification process involving a catalyst, carbon monoxide, hydrogen, steam and coal produces
A) only CO
B) liquid petroleum
C) octane
D) methane and carbon dioxide
A) only CO
B) liquid petroleum
C) octane
D) methane and carbon dioxide

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
32
The octane rating of straight run gasoline from fractional distillation of petroleum can be raised by
A) catalytic reforming
B) adding tertiary-butyl alcohol
C) adding ethanol
D) all of the above
A) catalytic reforming
B) adding tertiary-butyl alcohol
C) adding ethanol
D) all of the above

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
33
The combustible products of most coal gasification techniques are
A) nitrogen, hydrogen, and carbon monoxide
B) nitrogen and hydrogen
C) hydrogen and carbon monoxide
D) carbon dioxide and hydrogen
A) nitrogen, hydrogen, and carbon monoxide
B) nitrogen and hydrogen
C) hydrogen and carbon monoxide
D) carbon dioxide and hydrogen

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is not true about the compound MTBE?
A) it is an ether
B) made by fermentation
C) it is a carcinogen
D) used to oxygenate gasoline
A) it is an ether
B) made by fermentation
C) it is a carcinogen
D) used to oxygenate gasoline

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
35
Which substance is grain alcohol?
A) methanol
B) ethanol
C) MTBE
D) benzene
A) methanol
B) ethanol
C) MTBE
D) benzene

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
36
What are the bond angles around the carbon atoms in alkanes?
A) 90
B) l09.5
C) l20
D) l80
A) 90
B) l09.5
C) l20
D) l80

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
37
Gasoline is a
A) single, simple hydrocarbon
B) single, complex hydrocarbon
C) mixture of C12-C16 hydrocarbons
D) mixture of C5-C12 hydrocarbons
A) single, simple hydrocarbon
B) single, complex hydrocarbon
C) mixture of C12-C16 hydrocarbons
D) mixture of C5-C12 hydrocarbons

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
38
Petroleum is separated into fractions in the refining process.Which of the following fractions has molecules with the largest size?
A) gas
B) kerosene
C) paraffin wax
D) straight-run gasoline
A) gas
B) kerosene
C) paraffin wax
D) straight-run gasoline

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
39
The purpose of coal gasification is to
A) pressurize coal so the coal will be compact
B) produce a relatively clean burning, gaseous fuel
C) make more consumer products from coal
D) put railroads out of business
A) pressurize coal so the coal will be compact
B) produce a relatively clean burning, gaseous fuel
C) make more consumer products from coal
D) put railroads out of business

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
40
Octane ratings are based on comparison of the fuel being tested with the knocking properties of mixtures of
A) hexane and heptane
B) isooctane and hexane
C) isooctane and heptane
D) heptane and nonane
A) hexane and heptane
B) isooctane and hexane
C) isooctane and heptane
D) heptane and nonane

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
41
Identify the alkene?
A) C5H12
B) C10H20
C) C3H4
D) C18H40
A) C5H12
B) C10H20
C) C3H4
D) C18H40

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
42
Identify the alkane?
A) C5H12
B) C10H20
C) C3H4
D) C18H40
A) C5H12
B) C10H20
C) C3H4
D) C18H40

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
43
Which fuel has the largest heat of combustion per gram?
A) hydrogen
B) ethanol
C) wood
D) gasoline
A) hydrogen
B) ethanol
C) wood
D) gasoline

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
44
Which hydrocarbon would be isolated at the top of a fractionation column?
A) C5H12
B) C10H22
C) C3H8
D) C18H36
A) C5H12
B) C10H22
C) C3H8
D) C18H36

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
45
Identify the strongest bond?
A) C-C
B) C-H
C) C-O
D) C=O
A) C-C
B) C-H
C) C-O
D) C=O

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
46
What term is used to describe the amount of heat energy released from burning?
A) heat of fusion
B) heat of vaporization
C) heat of combustion
D) heat of formation
A) heat of fusion
B) heat of vaporization
C) heat of combustion
D) heat of formation

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck


