Deck 4: Carbon: the Basis of Molecular Diversity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 4: Carbon: the Basis of Molecular Diversity
1
Compared to a hydrocarbon chain where all the carbon atoms are linked by single bonds, which of the following statements best describe a hydrocarbon chain with the same number of carbon atoms but with one or more double bonds?
A) It will be more flexible in structure.
B) It will be more constrained in structure.
C) It will be more polar.
D) It will contain more hydrogen atoms.
A) It will be more flexible in structure.
B) It will be more constrained in structure.
C) It will be more polar.
D) It will contain more hydrogen atoms.
B
2
Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers; what are enantiomers?
A) Molecules that have identical chemical formulas but differ in the branching of their carbon skeletons.
B) Molecules that are mirror images of each other.
C) Molecules that differ in the location of their double bonds.
D) Molecules that differ in the arrangement of atoms around their double bonds.
A) Molecules that have identical chemical formulas but differ in the branching of their carbon skeletons.
B) Molecules that are mirror images of each other.
C) Molecules that differ in the location of their double bonds.
D) Molecules that differ in the arrangement of atoms around their double bonds.
B
3
Miller's classic experiment demonstrated that a discharge of sparks through a mixture of gases could result in the formation of a large variety of organic compounds. Miller did not use which one of the following gases in his experiment?
A) methane
B) oxygen
C) water
D) ammonia
A) methane
B) oxygen
C) water
D) ammonia
B
4
Which of the following factors determines whether a carbon atom's covalent bonds with other atoms are in a tetrahedral configuration or a planar configuration?
A) temperature of the solvent
B) double bonds between the carbon atom and other atoms
C) polarity of the covalent bonds
D) solvent in which the organic molecule is dissolved
A) temperature of the solvent
B) double bonds between the carbon atom and other atoms
C) polarity of the covalent bonds
D) solvent in which the organic molecule is dissolved

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
Thalidomide was sold as "racemic mixture" to pregnant women to treat morning sickness during pregnancy but the drug was found to harm fetus in the womb. It was banned in 1962. Which of the following reasons is the best explanation of the harmful effects of the drug?
A) Racemic mixture of any compound is always toxic and should be avoided.
B) (+)(R)-thalidomide is a sedative, but (-)(S)-thalidomide is a teratogen (a drug that is toxic in nature).
C) Thalidomide is not isomeric in nature thus causes adverse events.
D) Thalidomide was under dosed.
A) Racemic mixture of any compound is always toxic and should be avoided.
B) (+)(R)-thalidomide is a sedative, but (-)(S)-thalidomide is a teratogen (a drug that is toxic in nature).
C) Thalidomide is not isomeric in nature thus causes adverse events.
D) Thalidomide was under dosed.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following accurately describes a characteristic of isomers?
A) Isomers differ in both physical and chemical properties.
B) Isomers have same molecular formula.
C) Isomers are not structurally different.
D) Geometric and Optical isomerism are two types of stereoisomerism.
A) Isomers differ in both physical and chemical properties.
B) Isomers have same molecular formula.
C) Isomers are not structurally different.
D) Geometric and Optical isomerism are two types of stereoisomerism.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
Which statement is true about the following molecule? 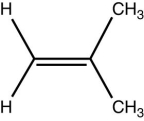
A) It is a cis isomer.
B) It is a trans isomer.
C) It is cis in its vapor form and trans in its fluid form.
D) It is not an isomer.

A) It is a cis isomer.
B) It is a trans isomer.
C) It is cis in its vapor form and trans in its fluid form.
D) It is not an isomer.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following characteristics is responsible for the complexity and variety of organic molecules?
A) the chemical versatility of carbon atoms
B) the variety of rare elements in organic molecules
C) the diverse bonding patterns of nitrogen
D) their interaction with water
A) the chemical versatility of carbon atoms
B) the variety of rare elements in organic molecules
C) the diverse bonding patterns of nitrogen
D) their interaction with water

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is correct for organic molecules with only hydrogens and five carbon atoms?
A) only a linear carbon skeleton is possible for the molecule
B) only carbon number 1 and 2 can form a double bond between them
C) different positions of double bonds between carbon atoms is not possible
D) the molecule cannot have enantiomers
A) only a linear carbon skeleton is possible for the molecule
B) only carbon number 1 and 2 can form a double bond between them
C) different positions of double bonds between carbon atoms is not possible
D) the molecule cannot have enantiomers

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following characteristics of an atom determines the number and kind of bonds it can form?
A) its atomic number
B) the number of particles in its valence shell
C) its atomic mass
D) the number of particles in its nucleus
A) its atomic number
B) the number of particles in its valence shell
C) its atomic mass
D) the number of particles in its nucleus

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following molecules has the fewest characteristics of an organic molecule?
A) water
B) methane
C) keratin-fibrous protein forming the main structure of hair
D) hemoglobin-iron-containing oxygen transport metalloprotein
A) water
B) methane
C) keratin-fibrous protein forming the main structure of hair
D) hemoglobin-iron-containing oxygen transport metalloprotein

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following unique feature of carbon allows it to support life on Earth?
A) Carbon is hydrophobic in nature.
B) Carbon is the most abundant element on earth.
C) Carbon can form a variety of bonds in nature.
D) Carbon is the most electronegative element in the periodic table.
A) Carbon is hydrophobic in nature.
B) Carbon is the most abundant element on earth.
C) Carbon can form a variety of bonds in nature.
D) Carbon is the most electronegative element in the periodic table.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
How many electrons must one carbon atom share with other atom or atoms to complete its valence shell?
A) two
B) three
C) four
D) eight
A) two
B) three
C) four
D) eight

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is true of carbon?
A) It forms only polar molecules.
B) It can form a maximum of three covalent bonds with other elements.
C) It is highly electronegative.
D) It can form both polar and nonpolar bonds.
A) It forms only polar molecules.
B) It can form a maximum of three covalent bonds with other elements.
C) It is highly electronegative.
D) It can form both polar and nonpolar bonds.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
Differences in which of the following chemical characteristics are responsible for the uniqueness of individuals organisms?
A) elemental composition from organism to organism
B) types and relative amounts of organic molecules synthesized by each organism
C) sizes of the organic molecules in each organism
D) types of inorganic compounds present in each organism
A) elemental composition from organism to organism
B) types and relative amounts of organic molecules synthesized by each organism
C) sizes of the organic molecules in each organism
D) types of inorganic compounds present in each organism

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
The maximum number of hydrogen atoms in an alkane with six carbons is ________.
A) 6
B) 10
C) 12
D) 14
A) 6
B) 10
C) 12
D) 14

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
The results from Stanley Miller's 1953 experiments can best be used to support which of the following hypotheses?
A) life on Earth arose from simple inorganic molecules
B) organic molecules can be synthesized abiotically under conditions that may have existed on early Earth
C) life on Earth arose from simple organic molecules, with energy from lightning and volcanoes
D) the conditions on early Earth were conducive to the origin of life
A) life on Earth arose from simple inorganic molecules
B) organic molecules can be synthesized abiotically under conditions that may have existed on early Earth
C) life on Earth arose from simple organic molecules, with energy from lightning and volcanoes
D) the conditions on early Earth were conducive to the origin of life

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following explains why the valency of carbon is 4 even though it has 6 electrons?
A) donates its 2 electrons to another atom
B) shares its 2 electrons and bonds with another atom
C) has 4 electrons in its first shell and 2 in the second shell
D) has 2 electrons in its first shell and 4 in the second shell
A) donates its 2 electrons to another atom
B) shares its 2 electrons and bonds with another atom
C) has 4 electrons in its first shell and 2 in the second shell
D) has 2 electrons in its first shell and 4 in the second shell

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following structures is a correct representation of an alcohol?
A)
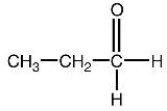
B)
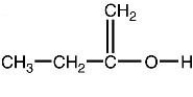
C)
D)
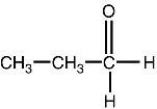
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
A carbon atom is most likely to form what kind of bond(s) with other atoms?
A) ionic
B) hydrogen
C) covalent
D) carbon is an inert element
A) ionic
B) hydrogen
C) covalent
D) carbon is an inert element

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
Use the figures to answer the question. 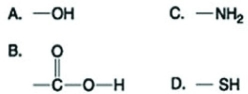 Which functional group shown in the figure can accept protons and raise the pH of the surrounding solution?
Which functional group shown in the figure can accept protons and raise the pH of the surrounding solution?
A) A
B) B
C) C
D) D
 Which functional group shown in the figure can accept protons and raise the pH of the surrounding solution?
Which functional group shown in the figure can accept protons and raise the pH of the surrounding solution?A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
Use the figures to answer the following question. 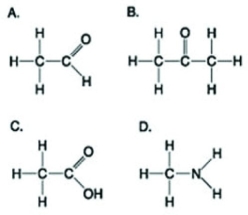 Which molecule shown has at least one carbon atom attached to three different chemical groups?
Which molecule shown has at least one carbon atom attached to three different chemical groups?
A) A
B) B
C) D
D) A and B
 Which molecule shown has at least one carbon atom attached to three different chemical groups?
Which molecule shown has at least one carbon atom attached to three different chemical groups?A) A
B) B
C) D
D) A and B

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
Use the following figure to answer the question. 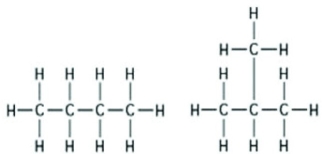 The two molecules shown in the figures are best described as ________.
The two molecules shown in the figures are best described as ________.
A) enantiomers
B) structural isomers
C) cis-trans isomers
D) chain length isomers
 The two molecules shown in the figures are best described as ________.
The two molecules shown in the figures are best described as ________.A) enantiomers
B) structural isomers
C) cis-trans isomers
D) chain length isomers

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
Use the figures to answer the question. 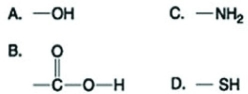 Which of the functional groups shown in the figure is present in hexanol but not in hexane?
Which of the functional groups shown in the figure is present in hexanol but not in hexane?
A) A
B) B
C) C
D) D
 Which of the functional groups shown in the figure is present in hexanol but not in hexane?
Which of the functional groups shown in the figure is present in hexanol but not in hexane?A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
Which two functional groups are always found in amino acids?
A) carbonyl and amino groups
B) carboxyl and amino groups
C) amino and sulfhydryl groups
D) hydroxyl and carboxyl groups
A) carbonyl and amino groups
B) carboxyl and amino groups
C) amino and sulfhydryl groups
D) hydroxyl and carboxyl groups

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
A compound contains hydroxyl groups as its predominant functional group. Which of the following properties of the molecule can be predicted with the information provided?
A) It lacks an asymmetric carbon and is probably a fat or lipid.
B) It should dissolve in water.
C) It should dissolve in a nonpolar solvent.
D) It will not form hydrogen bonds with water.
A) It lacks an asymmetric carbon and is probably a fat or lipid.
B) It should dissolve in water.
C) It should dissolve in a nonpolar solvent.
D) It will not form hydrogen bonds with water.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the pairs of molecular structures shown depict enantiomers (enantiomeric forms) of the same molecule?
A)
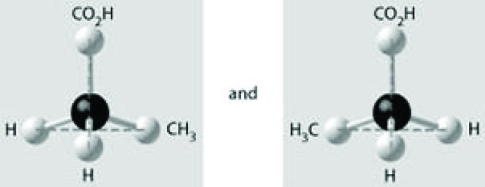
B)
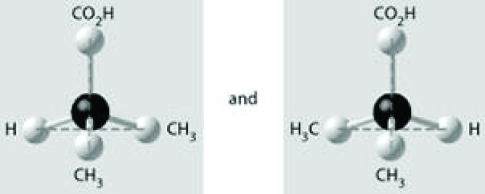
C)
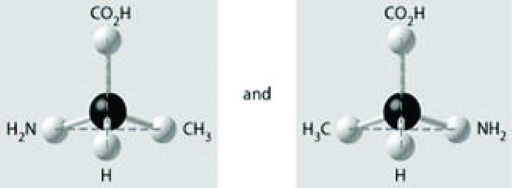
D)
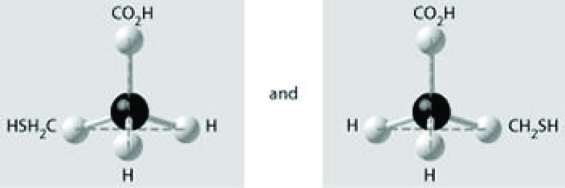
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following functional groups is hydrophobic in nature?
A) hydroxyl
B) methyl
C) amino
D) sulfhydryl
A) hydroxyl
B) methyl
C) amino
D) sulfhydryl

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
Use the figures to answer the following question. 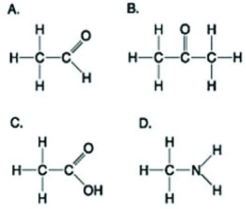 Which molecule shown has a carbonyl functional group in the form of a ketone?
Which molecule shown has a carbonyl functional group in the form of a ketone?
A) A
B) B
C) C
D) D
 Which molecule shown has a carbonyl functional group in the form of a ketone?
Which molecule shown has a carbonyl functional group in the form of a ketone?A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following functional groups gives amino acids their acidic character?
A) amino
B) carbonyl
C) carboxyl
D) phosphate
A) amino
B) carbonyl
C) carboxyl
D) phosphate

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following functional groups is unreactive but when added to bases of DNA can alter gene expression?
A) amino
B) methyl
C) carboxyl
D) hydroxyl
A) amino
B) methyl
C) carboxyl
D) hydroxyl

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In which of the following ways do these molecules differ from each other?
A) They are structural isomers but have the same molecular formula.
B) They are cis-trans isomers but have the same molecular formula.
C) They have different functional groups attached to the same carbon skeleton.
D) They are enantiomers of the same organic molecule.
A) They are structural isomers but have the same molecular formula.
B) They are cis-trans isomers but have the same molecular formula.
C) They have different functional groups attached to the same carbon skeleton.
D) They are enantiomers of the same organic molecule.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
What is the relationship between the following two molecules? 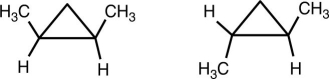
A) They are enantiomers.
B) They are not isomers.
C) They are geometrical isomers.
D) They are the same molecule.
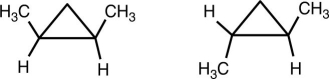
A) They are enantiomers.
B) They are not isomers.
C) They are geometrical isomers.
D) They are the same molecule.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
Use the following figure to answer the following question. 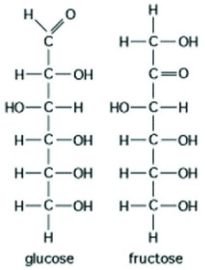 The figure shows the structures of glucose and fructose. Which of the following describes a difference between the two molecules?
The figure shows the structures of glucose and fructose. Which of the following describes a difference between the two molecules?
A) number of carbon, hydrogen, and oxygen atoms
B) types of carbon, hydrogen, and oxygen atoms
C) arrangement of carbon, hydrogen, and oxygen atoms
D) number of oxygen atoms joined to carbon atoms by double covalent bonds
 The figure shows the structures of glucose and fructose. Which of the following describes a difference between the two molecules?
The figure shows the structures of glucose and fructose. Which of the following describes a difference between the two molecules?A) number of carbon, hydrogen, and oxygen atoms
B) types of carbon, hydrogen, and oxygen atoms
C) arrangement of carbon, hydrogen, and oxygen atoms
D) number of oxygen atoms joined to carbon atoms by double covalent bonds

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following identifies the chemical relationship between glucose and fructose?
A) They are isotopes.
B) They are enantiomers.
C) They are cis-trans isomers.
D) They are structural isomers.
A) They are isotopes.
B) They are enantiomers.
C) They are cis-trans isomers.
D) They are structural isomers.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
Use the figures to answer the following question. 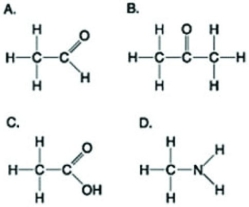 Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
A) A
B) B
C) C
D) D
 Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?
Which molecule shown can increase the concentration of hydrogen ions in a solution and is therefore an organic acid?A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following functional groups is present in acetic acid?
A) hydroxyl group
B) sulfhydryl group
C) carbonyl group
D) carboxyl group
A) hydroxyl group
B) sulfhydryl group
C) carbonyl group
D) carboxyl group

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following illustrations is not a structural isomer of an organic compound with the molecular formula C6H14? For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted.
A)
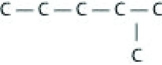
B)
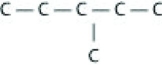
C)
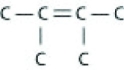
D)
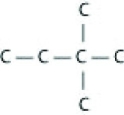
A)
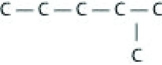
B)
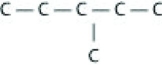
C)
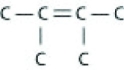
D)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
Use the figures to answer the following question. 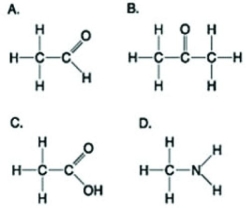 Which molecule(s) shown is (are) ionized in a cell?
Which molecule(s) shown is (are) ionized in a cell?
A) A
B) B and D
C) C and D
D) D
 Which molecule(s) shown is (are) ionized in a cell?
Which molecule(s) shown is (are) ionized in a cell?A) A
B) B and D
C) C and D
D) D

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following compounds is found in vinegar?
A) nitric acid
B) acetic acid
C) amino acid
D) propionic acid
A) nitric acid
B) acetic acid
C) amino acid
D) propionic acid

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
Which chemical group is most likely to be responsible for an organic molecule behaving as a base?
A) hydroxyl
B) carbonyl
C) amino
D) phosphate
A) hydroxyl
B) carbonyl
C) amino
D) phosphate

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
Which chemical change will convert ADP to ATP?
A) by adding energy and a phosphate
B) by adding two phosphates
C) by removing energy and a phosphate
D) by adding a phosphate, energy and oxygen
A) by adding energy and a phosphate
B) by adding two phosphates
C) by removing energy and a phosphate
D) by adding a phosphate, energy and oxygen

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
Hydrolysis of ADP produces which of the following products?
A) AMP + Pi + energy
B) ATP + Pi
C) Pi + Pi + water
D) ATP + energy
A) AMP + Pi + energy
B) ATP + Pi
C) Pi + Pi + water
D) ATP + energy

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
ATP is necessary for life because ________.
A) it tastes good
B) it is soluble in water
C) it speeds up the biological processes
D) it is the principle energy carrying molecule in a cell
A) it tastes good
B) it is soluble in water
C) it speeds up the biological processes
D) it is the principle energy carrying molecule in a cell

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
Use the figures to answer the following question. 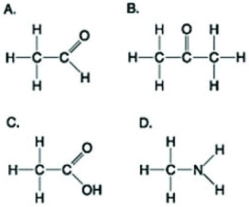 Which molecule shown can be a result of mercaptoethanol reduction of a disulfide bridge?
Which molecule shown can be a result of mercaptoethanol reduction of a disulfide bridge?
A) A
B) B
C) C
D) D
 Which molecule shown can be a result of mercaptoethanol reduction of a disulfide bridge?
Which molecule shown can be a result of mercaptoethanol reduction of a disulfide bridge?A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
Use the figures to answer the question. 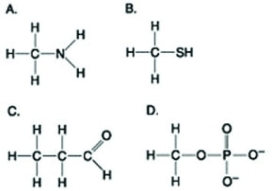 Which molecule shown above contains a functional group that is a part of the principal molecule that stores and transfers energy in cells?
Which molecule shown above contains a functional group that is a part of the principal molecule that stores and transfers energy in cells?
A) A
B) B
C) C
D) D
 Which molecule shown above contains a functional group that is a part of the principal molecule that stores and transfers energy in cells?
Which molecule shown above contains a functional group that is a part of the principal molecule that stores and transfers energy in cells?A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following statements about ADP/ATP is true?
A) ADP contains more energy than ATP.
B) Following hydrolysis, ATP can release one phosphate, whereas ADP cannot.
C) ADP can have two positive charges.
D) ATP can have four negative charges.
A) ADP contains more energy than ATP.
B) Following hydrolysis, ATP can release one phosphate, whereas ADP cannot.
C) ADP can have two positive charges.
D) ATP can have four negative charges.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
Organic chemistry is currently defined as ________.
A) the study of compounds made only by living cells
B) the study of carbon compounds
C) the study of natural (as opposed to synthetic) compounds
D) the study of hydrocarbons
A) the study of compounds made only by living cells
B) the study of carbon compounds
C) the study of natural (as opposed to synthetic) compounds
D) the study of hydrocarbons

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
Which functional group is present in this molecule? 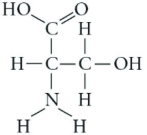
A) sulfhydryl
B) carboxyl
C) methyl
D) phosphate

A) sulfhydryl
B) carboxyl
C) methyl
D) phosphate

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
Which action could produce a carbonyl group?
A) the replacement of the -OH of a carboxyl group with hydrogen
B) the addition of a thiol to a hydroxyl
C) the addition of a hydroxyl to a phosphate
D) the replacement of the nitrogen of an amine with oxygen
A) the replacement of the -OH of a carboxyl group with hydrogen
B) the addition of a thiol to a hydroxyl
C) the addition of a hydroxyl to a phosphate
D) the replacement of the nitrogen of an amine with oxygen

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
Choose the term that correctly describes the relationship between these two sugar molecules. 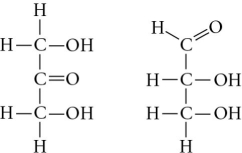
A) structural isomers
B) cis-trans isomers
C) enantiomers
D) isotopes

A) structural isomers
B) cis-trans isomers
C) enantiomers
D) isotopes

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
Visualize the structural formula of each of the following hydrocarbons. Which hydrocarbon has a double bond in its carbon skeleton?
A) C3H8
B) C2H6
C) C2H4
D) C2H2
A) C3H8
B) C2H6
C) C2H4
D) C2H2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


