Deck 2: Atoms and Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/61
Play
Full screen (f)
Deck 2: Atoms and Molecules
1
Molybdenum has an atomic number of 42. Several common isotopes exist, with mass numbers from 92-100. Based on this information, which of the following is also true of molybdenum?
A) Molybdenum atoms can have between 50 and 58 neutrons.
B) Molybdenum atoms can have between 50 and 58 protons.
C) Molybdenum atoms can have between 50 and 58 electrons.
D) Isotopes of molybdenum have different numbers of electrons.
A) Molybdenum atoms can have between 50 and 58 neutrons.
B) Molybdenum atoms can have between 50 and 58 protons.
C) Molybdenum atoms can have between 50 and 58 electrons.
D) Isotopes of molybdenum have different numbers of electrons.
A
2
 How many electrons are present in the neutral atom represented in the Periodic Table block in the figure?
How many electrons are present in the neutral atom represented in the Periodic Table block in the figure?A) 18
B) 19
C) 22
D) 40
C
3
What element does not react with other elements?
A) hydrogen
B) helium
C) oxygen
D) silicon
A) hydrogen
B) helium
C) oxygen
D) silicon
B
4
Atoms have no electric charge because they have ________.
A) uncharged neutrons in their nuclei
B) an equal number of protons and neutrons
C) an equal number of protons and electrons
D) an equal number of charged and uncharged subatomic particles
A) uncharged neutrons in their nuclei
B) an equal number of protons and neutrons
C) an equal number of protons and electrons
D) an equal number of charged and uncharged subatomic particles

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
5
A neutral atom has 2, 8, 8 electrons in its first, second, and third energy levels. This information ________.
A) does not tell us about the atomic number of the element
B) does not tell us about the chemical properties of the element
C) does not tell us about the atomic mass of the element
D) does not tell us about the size of the element
A) does not tell us about the atomic number of the element
B) does not tell us about the chemical properties of the element
C) does not tell us about the atomic mass of the element
D) does not tell us about the size of the element

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements is TRUE?
A) Carbon, hydrogen, oxygen, and calcium are the most abundant elements of living matter.
B) Some naturally occurring elements are toxic to organisms.
C) All life requires the same essential elements.
D) A patient suffering from a goiter should not consume seafood.
A) Carbon, hydrogen, oxygen, and calcium are the most abundant elements of living matter.
B) Some naturally occurring elements are toxic to organisms.
C) All life requires the same essential elements.
D) A patient suffering from a goiter should not consume seafood.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
7
An ion that consists of 7 protons, 6 neutrons, and 11 electrons has a net charge of ________.
A) 4-
B) 5+
C) 5-
D) 4+
A) 4-
B) 5+
C) 5-
D) 4+

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
8
Which pair of elements is most likely to react if bought together?
A) hydrogen and argon
B) sodium and chlorine
C) hydrogen and lithium
D) nitrogen and oxygen
A) hydrogen and argon
B) sodium and chlorine
C) hydrogen and lithium
D) nitrogen and oxygen

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
9
 What is the atomic number of the neutral atom represented by the Periodic Table block in the figure?
What is the atomic number of the neutral atom represented by the Periodic Table block in the figure?A) 18
B) 19
C) 22
D) 39

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following has the smallest total mass?
A) two electrons
B) two neutrons
C) 1 electron plus 1 neutron
D) 1 neutron plus 1 proton
A) two electrons
B) two neutrons
C) 1 electron plus 1 neutron
D) 1 neutron plus 1 proton

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
11
How many electrons are present in a H- and H+ ion respectively?
A) 1,2
B) 2,1
C) 2,0
D) 0,2
A) 1,2
B) 2,1
C) 2,0
D) 0,2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
12
 How many electrons are present in the +2 ionic form of the atom in the Periodic Table block shown in the figure?
How many electrons are present in the +2 ionic form of the atom in the Periodic Table block shown in the figure?A) 18
B) 19
C) 22
D) 40

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following best explain why argon, which is a noble gas, does not react with other elements. Noble gases ________.
A) have completely paired up and stable electron shells
B) have very small atoms
C) are not found in abundance on our planet
D) have a high positive charge that repels most elements
A) have completely paired up and stable electron shells
B) have very small atoms
C) are not found in abundance on our planet
D) have a high positive charge that repels most elements

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
14
An ion with six protons, seven neutrons, and a charge of 2+ has an atomic number of ________.
A) four
B) five
C) six
D) seven
A) four
B) five
C) six
D) seven

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
15
Trace elements are those required by an organism in only minute quantities. Which of the following is a trace element that is required by all forms of life?
A) arsenic
B) iodine
C) mercury
D) iron
A) arsenic
B) iodine
C) mercury
D) iron

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
16
The atomic number of nitrogen is 7. Which of the following explains the greater mass number of nitrogen-15 compared to nitrogen-14? Nitrogen-15 contains ________.
A) 7 neutrons and nitrogen-14 contains 8 neutrons
B) 8 neutrons and nitrogen-14 contains 7 neutrons
C) 8 protons and nitrogen 14 contains 7 protons
D) 15 protons and nitrogen-14 contains 14 protons
A) 7 neutrons and nitrogen-14 contains 8 neutrons
B) 8 neutrons and nitrogen-14 contains 7 neutrons
C) 8 protons and nitrogen 14 contains 7 protons
D) 15 protons and nitrogen-14 contains 14 protons

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
17
About 25 of the 92 natural elements are known to be essential to life. Four of these 25 elements make up approximately 96% of living matter. Which of the following elements account for most of the remaining 4% of an organism's mass?
A) carbon, oxygen, hydrogen, nitrogen
B) calcium, potassium, phosphorus, sulfur
C) oxygen, hydrogen, calcium, nitrogen
D) carbon, hydrogen, nitrogen, oxygen
A) carbon, oxygen, hydrogen, nitrogen
B) calcium, potassium, phosphorus, sulfur
C) oxygen, hydrogen, calcium, nitrogen
D) carbon, hydrogen, nitrogen, oxygen

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is true of oxygen that has 8 protons, 8 neutrons, and 8 electrons?
A) It has a charge of +8.
B) It has a mass number of 8.
C) It has an atomic number of 8.
D) It has atomic number of 16.
A) It has a charge of +8.
B) It has a mass number of 8.
C) It has an atomic number of 8.
D) It has atomic number of 16.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following atoms has the smallest number of neutrons?
A) nitrogen-14
B) carbon-14
C) oxygen-16
D) neon-20
A) nitrogen-14
B) carbon-14
C) oxygen-16
D) neon-20

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following are compounds?
A) H2O, O2, and CH4
B) H2O and O2
C) O2 and CH4
D) H2O and CH4, but not O2
A) H2O, O2, and CH4
B) H2O and O2
C) O2 and CH4
D) H2O and CH4, but not O2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
21
The atomic number of chlorine is 17. The atomic number of magnesium is 12. Given this information, what is the formula for magnesium chloride?
A) MgCl
B) MgCl2
C) Mg2Cl
D) MgCl3
A) MgCl
B) MgCl2
C) Mg2Cl
D) MgCl3

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
22
A covalent bond is likely to be polar under which of the following conditions?
A) one of the atoms sharing electrons is more electronegative than the other atom
B) the two atoms sharing electrons are equally electronegative
C) carbon is one of the two atoms sharing electrons
D) the two atoms sharing electrons are of the same elements
A) one of the atoms sharing electrons is more electronegative than the other atom
B) the two atoms sharing electrons are equally electronegative
C) carbon is one of the two atoms sharing electrons
D) the two atoms sharing electrons are of the same elements

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
23
How many electrons will a single atom of nitrogen with no charge and no bonds have in its valence shell?
A) 2
B) 5
C) 7
D) 14
A) 2
B) 5
C) 7
D) 14

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
24
Which pair of elements in the diagram is most likely to form a covalent bond?
A) V and Z
B) V and Y
C) V and X
D) W and Z
A) V and Z
B) V and Y
C) V and X
D) W and Z

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following models represents an atom that is most likely to form a cation with a charge of +1?
A)
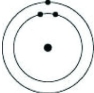
B)
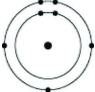
C)
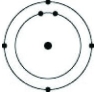
D)
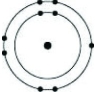
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
26
Nitrogen (N) is more electronegative than hydrogen (H). Which of the following is a correct statement about the atoms in ammonia (NH3)?
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) Ammonia has an overall positive charge.
C) Ammonia has an overall negative charge.
D) The nitrogen atom has a partial positive charge; each hydrogen atom has a partial negative charge.
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) Ammonia has an overall positive charge.
C) Ammonia has an overall negative charge.
D) The nitrogen atom has a partial positive charge; each hydrogen atom has a partial negative charge.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
27
A salamander relies on hydrogen bonding to stick to various surfaces. Therefore, a salamander would have the greatest difficulty clinging to a ________.
A) slightly damp surface
B) surface of hydrocarbons
C) surface of mostly carbon-oxygen bonds
D) surface of mostly carbon-nitrogen bonds
A) slightly damp surface
B) surface of hydrocarbons
C) surface of mostly carbon-oxygen bonds
D) surface of mostly carbon-nitrogen bonds

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
28
In the following structure where A and B represent two different elements, the valency of A is ________ and B is ________. 
A) one; three
B) one; five
C) three; five
D) eight; eight

A) one; three
B) one; five
C) three; five
D) eight; eight

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
29
Elements 72Zn, 75As, and 74Ge have the same number of ________.
A) protons
B) protons and electrons
C) neutrons
D) neutrons and electrons
A) protons
B) protons and electrons
C) neutrons
D) neutrons and electrons

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
30
To find out the number of neutrons in an atom, we need to know the following.
A) atomic number
B) electron number
C) mass number
D) mass and atomic number
A) atomic number
B) electron number
C) mass number
D) mass and atomic number

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following types of bond is broken when water evaporates?
A) nonpolar covalent bonds
B) ionic bonds
C) hydrogen bonds
D) polar covalent bonds
A) nonpolar covalent bonds
B) ionic bonds
C) hydrogen bonds
D) polar covalent bonds

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
32
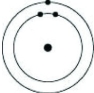
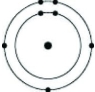
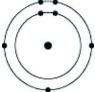
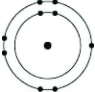
Which of the following models represents an atom that is most likely to form an anion with a charge of -1?
A)
B)
C)
D)
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
33
Under which of the following conditions will an atom be the most stable?
A) when they have the fewest possible valence electrons
B) when they have the maximum number of unpaired electrons
C) when all of the electron orbitals in the valence shell are filled
D) when all electrons are paired
A) when they have the fewest possible valence electrons
B) when they have the maximum number of unpaired electrons
C) when all of the electron orbitals in the valence shell are filled
D) when all electrons are paired

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
34
Considering that the reactants have no charge, what are the products of the reaction shown above?
A) a cation with a net charge of +1 and an anion with a net charge of +1
B) a cation with a net charge of -1 and an anion with a net charge of -1
C) a cation with a net charge of -1 and an anion with a net charge of +1
D) a cation with a net charge of +1 and an anion with a net charge of -1
A) a cation with a net charge of +1 and an anion with a net charge of +1
B) a cation with a net charge of -1 and an anion with a net charge of -1
C) a cation with a net charge of -1 and an anion with a net charge of +1
D) a cation with a net charge of +1 and an anion with a net charge of -1

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
35
How many electron pairs are shared between carbon atoms in a molecule that has the formula C2H4?
A) one
B) two
C) three
D) four
A) one
B) two
C) three
D) four

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
36
In the above diagram, what kind of bond is most likely to form between V and Z?
A) ionic
B) covalent
C) hydrogen
D) van der Waals
A) ionic
B) covalent
C) hydrogen
D) van der Waals

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
37
Van der Waals interactions may result under which of the following conditions?
A) electrons are not symmetrically distributed in a molecule
B) molecules held by ionic bonds react with water
C) two polar covalent bonds react
D) a hydrogen atom loses an electron
A) electrons are not symmetrically distributed in a molecule
B) molecules held by ionic bonds react with water
C) two polar covalent bonds react
D) a hydrogen atom loses an electron

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
38
Based on electron configuration, which of the elements would exhibit a chemical behavior similar to oxygen?
A) carbon
B) nitrogen
C) sulfur
D) phosphorus
A) carbon
B) nitrogen
C) sulfur
D) phosphorus

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
39
How many electrons are present in a Phosphorus 2+ atom?
A) 12
B) 13
C) 19
D) 34
A) 12
B) 13
C) 19
D) 34

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
40
Oxygen has an atomic number of 8 and, most commonly, a mass number of 16. Thus, what is the atomic mass of an oxygen atom?
A) approximately 8 grams
B) approximately 8 daltons
C) approximately 16 grams
D) approximately 16 daltons
A) approximately 8 grams
B) approximately 8 daltons
C) approximately 16 grams
D) approximately 16 daltons

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
41
How many electrons participate in a triple covalent bond?
A) 3
B) 6
C) 9
D) 12
A) 3
B) 6
C) 9
D) 12

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following statements correctly describes any chemical reaction that has reached equilibrium?
A) The concentrations of products and reactants are equal.
B) The reaction is now irreversible.
C) Both forward and reverse reactions have halted.
D) The rates of the forward and reverse reactions are equal.
A) The concentrations of products and reactants are equal.
B) The reaction is now irreversible.
C) Both forward and reverse reactions have halted.
D) The rates of the forward and reverse reactions are equal.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
43
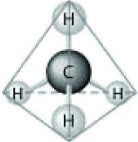 Which of the following factors contribute to the tetrahedral shape of the above molecule?
Which of the following factors contribute to the tetrahedral shape of the above molecule?A) the shape of the two p orbitals in the carbon atom
B) the shape of the one s orbital in the carbon atom
C) the shape of the sp3 hybrid orbitals of the electrons shared between the carbon and hydrogen atoms
D) hydrogen bonding configurations between the carbon and hydrogen atoms

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
44
An atom has four electrons in its valence shell. What types of covalent bonds is it capable of forming?
A) single, double, or triple
B) single and double only
C) single bonds only
D) double bonds only
A) single, double, or triple
B) single and double only
C) single bonds only
D) double bonds only

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
45
The atomic number of sulfur is 16. Sulfur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulfur atom, predict the molecular formula of the compound.
A) HS
B) HS2
C) H2S
D) H4S
A) HS
B) HS2
C) H2S
D) H4S

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
46
Which one of the following describes the correct trends in electronegativity in the periodic table?
A) increases across a period and decreases down a group
B) decreases across a period and decreases down a group
C) increases across a period and increases down a group
D) decreases across a period and increases down a group
A) increases across a period and decreases down a group
B) decreases across a period and decreases down a group
C) increases across a period and increases down a group
D) decreases across a period and increases down a group

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following factors will increase the rate of reaction in the forward direction?
A) addition of nitrogen
B) addition of ammonia
C) addition of hydrogen
D) addition of both nitrogen and hydrogen
A) addition of nitrogen
B) addition of ammonia
C) addition of hydrogen
D) addition of both nitrogen and hydrogen

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
48
If an atom has a charge of +1, which of the following must be true?
A) It has two more protons than neutrons.
B) It has the same number of protons as electrons.
C) It has one more electron than it does protons.
D) It has one more proton than it does electrons.
A) It has two more protons than neutrons.
B) It has the same number of protons as electrons.
C) It has one more electron than it does protons.
D) It has one more proton than it does electrons.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
49
Which statement is true of all atoms that are anions?
A) The atom has more electrons than protons.
B) The atom has more protons than electrons.
C) The atom has fewer protons than does a neutral atom of the same element.
D) The atom has more neutrons than protons.
A) The atom has more electrons than protons.
B) The atom has more protons than electrons.
C) The atom has fewer protons than does a neutral atom of the same element.
D) The atom has more neutrons than protons.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
50
The reactivity of an atom arises from ________.
A) the average distance of the outermost electron shell from the nucleus
B) the existence of unpaired electrons in the valence shell
C) the sum of the potential energies of all the electron shells
D) the potential energy of the valence shell
A) the average distance of the outermost electron shell from the nucleus
B) the existence of unpaired electrons in the valence shell
C) the sum of the potential energies of all the electron shells
D) the potential energy of the valence shell

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
51
How is a covalent bond formed?
A) two atoms share two pairs of electrons
B) two atoms share two electrons
C) two atoms share one electron
D) one atom loses a pair of electrons to the other
A) two atoms share two pairs of electrons
B) two atoms share two electrons
C) two atoms share one electron
D) one atom loses a pair of electrons to the other

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following correctly describes chemical equilibrium?
A) Forward and reverse reactions continue with no net effect on the concentrations of the reactants and products.
B) Concentrations of products are higher than the concentrations of the reactants.
C) There are equal concentrations of products and reactants while forward and reverse reactions continue.
D) There are equal concentrations of reactants and products, and the reactions have stopped.
A) Forward and reverse reactions continue with no net effect on the concentrations of the reactants and products.
B) Concentrations of products are higher than the concentrations of the reactants.
C) There are equal concentrations of products and reactants while forward and reverse reactions continue.
D) There are equal concentrations of reactants and products, and the reactions have stopped.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following types of representation would work best to indicate the type and number of atoms in a molecule?
A) molecular formula
B) structural formula
C) ball-and-stick model
D) space-filling model
A) molecular formula
B) structural formula
C) ball-and-stick model
D) space-filling model

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
54
In the term trace element, the adjective trace means that ________.
A) the element is required in very small amounts
B) the element can be used as a label to trace atoms through an organism's metabolism
C) the element is very rare on Earth
D) the element enhances health but is not essential for the organism's long-term survival
A) the element is required in very small amounts
B) the element can be used as a label to trace atoms through an organism's metabolism
C) the element is very rare on Earth
D) the element enhances health but is not essential for the organism's long-term survival

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
55
What is the atomic number of the cation formed in the reaction in the illustration?
A) 8
B) 10
C) 11
D) 16
A) 8
B) 10
C) 11
D) 16

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
56
We can represent atoms by listing the number of protons, neutrons, and electrons-for example, 2p+, 2n0, 2e- for helium. Which of the following represents the 18O isotope of oxygen?
A) 7p+, 2n0, 9e-
B) 8p+, 10n0, 8e-
C) 9p+, 9n0, 9e-
D) 10p+, 8n0, 9e-
A) 7p+, 2n0, 9e-
B) 8p+, 10n0, 8e-
C) 9p+, 9n0, 9e-
D) 10p+, 8n0, 9e-

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
57
Elements found in the first two columns of the periodic table contain outer electron shells that are ________; these elements tend to form ________ in solution.
A) almost empty; cations
B) almost empty; anions
C) almost full; cations
D) almost full; anions
A) almost empty; cations
B) almost empty; anions
C) almost full; cations
D) almost full; anions

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
58
Nitrogen (N) normally forms three covalent bonds with a valence of five. However, ammonium has four covalent bonds, each to a different hydrogen (H) atom (H has a valence of one). What do you predict to be the charge on ammonium?
A) +1
B) -1
C) +2
D) -2
A) +1
B) -1
C) +2
D) -2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
59
Compared with 31P, the radioactive isotop 32P has ________.
A) a different atomic number
B) one more proton
C) one more electron
D) one more neutron
A) a different atomic number
B) one more proton
C) one more electron
D) one more neutron

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is true for the above reaction?
A) the reaction is nonreversible
B) hydrogen and nitrogen are the reactants of the reverse reaction
C) ammonia is being formed and decomposed simultaneously
D) only the forward or reverse reactions can occur at one time
A) the reaction is nonreversible
B) hydrogen and nitrogen are the reactants of the reverse reaction
C) ammonia is being formed and decomposed simultaneously
D) only the forward or reverse reactions can occur at one time

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
61
What coefficients must be placed in the following blanks so that all atoms are accounted for in the products?
C6H12O6 → ________ C2H6O + ________ CO2
A) 2; 1
B) 3; 1
C) 1; 3
D) 2; 2
C6H12O6 → ________ C2H6O + ________ CO2
A) 2; 1
B) 3; 1
C) 1; 3
D) 2; 2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck


