Deck 6: Energy and Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/65
Play
Full screen (f)
Deck 6: Energy and Life
1
Living organisms increase in complexity as they grow, resulting in a decrease in the entropy of an organism. How does this relate to the second law of thermodynamics?
A) Living organisms do not obey the second law of thermodynamics, which states that the entropy of an organism increases with each energy transformation.
B) As a consequence of the processes required for growth, the decrease in entropy of the organism is associated with a corresponding increase in the entropy of the universe.
C) As a consequence of the processes required for growth, the decrease in entropy of the organism is associated with a corresponding decrease in the entropy of the universe.
D) Living organisms are able to transform chemical energy into entropy.
A) Living organisms do not obey the second law of thermodynamics, which states that the entropy of an organism increases with each energy transformation.
B) As a consequence of the processes required for growth, the decrease in entropy of the organism is associated with a corresponding increase in the entropy of the universe.
C) As a consequence of the processes required for growth, the decrease in entropy of the organism is associated with a corresponding decrease in the entropy of the universe.
D) Living organisms are able to transform chemical energy into entropy.
B
2
A decrease in entropy is associated with which of the following types of reaction?
A) dehydration
B) catabolic
C) depolymerization
D) hydrolysis
A) dehydration
B) catabolic
C) depolymerization
D) hydrolysis
A
3
Which of the following statements best describes a system at chemical equilibrium?
A) The system consumes energy at a steady rate.
B) The system releases energy at a steady rate.
C) The kinetic energy of the system is zero.
D) The system can do no work.
A) The system consumes energy at a steady rate.
B) The system releases energy at a steady rate.
C) The kinetic energy of the system is zero.
D) The system can do no work.
D
4
Which of the following terms most precisely identifies the cellular process of breaking down large molecules into smaller ones?
A) catabolism (catabolic pathways)
B) metabolism
C) anabolism (anabolic pathways)
D) dehydration
A) catabolism (catabolic pathways)
B) metabolism
C) anabolism (anabolic pathways)
D) dehydration

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements best describes metabolism in its entirety in all organisms?
A) Metabolism depends on a constant supply of energy from food.
B) Metabolism uses all of an organism's resources.
C) Metabolism consists of all the energy transformation reactions in an organism.
D) Metabolism manages the increase of entropy in an organism.
A) Metabolism depends on a constant supply of energy from food.
B) Metabolism uses all of an organism's resources.
C) Metabolism consists of all the energy transformation reactions in an organism.
D) Metabolism manages the increase of entropy in an organism.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements best describes an example of a reaction that may be at chemical equilibrium in a cell?
A) an exergonic reaction in which the free energy at equilibrium is higher than the energy content of the reaction at any point away from equilibrium
B) an exergonic reaction in which the entropy change in the cell is precisely balanced by an opposite entropy change in the cell's surroundings
C) a chemical reaction in which there is no change in the net concentrations of reactants or products at that time in the cell
D) an endergonic reaction in an active metabolic pathway where the energy for that reaction is supplied only by heat from the environment
A) an exergonic reaction in which the free energy at equilibrium is higher than the energy content of the reaction at any point away from equilibrium
B) an exergonic reaction in which the entropy change in the cell is precisely balanced by an opposite entropy change in the cell's surroundings
C) a chemical reaction in which there is no change in the net concentrations of reactants or products at that time in the cell
D) an endergonic reaction in an active metabolic pathway where the energy for that reaction is supplied only by heat from the environment

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following terms best describes a chemical reaction for which ΔG is positive?
A) endergonic
B) enthalpic
C) spontaneous
D) exergonic
A) endergonic
B) enthalpic
C) spontaneous
D) exergonic

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements is consistent with the second law of thermodynamics?
A) A gain of free energy in a system is never associated with conversion of energy from one form to another.
B) A constant input of energy is required to maintain the high level of organization associated with living cells.
C) Without an input of energy, the entropy of an organism would tend to decrease over time.
D) Every energy transformation performed by an organism decreases the entropy of the universe.
A) A gain of free energy in a system is never associated with conversion of energy from one form to another.
B) A constant input of energy is required to maintain the high level of organization associated with living cells.
C) Without an input of energy, the entropy of an organism would tend to decrease over time.
D) Every energy transformation performed by an organism decreases the entropy of the universe.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
9
The relationship between the breakdown of macromolecules and the biosynthesis of macromolecules is most similar to the relationship between which of the following pairs of terms?
A) exergonic and spontaneous
B) exergonic and endergonic
C) free energy and entropy
D) work and free energy
A) exergonic and spontaneous
B) exergonic and endergonic
C) free energy and entropy
D) work and free energy

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following examples describes a type of potential rather than kinetic energy?
A) water rushing out of Hoover Dam
B) flashes of light emitted by a firefly
C) a sucrose molecule
D) heat released by a campfire
A) water rushing out of Hoover Dam
B) flashes of light emitted by a firefly
C) a sucrose molecule
D) heat released by a campfire

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements best describes an exergonic reaction?
A) The products have more total energy than the reactants.
B) The reaction proceeds with a net release of free energy.
C) The reaction goes only in a forward direction: all reactants will be converted to products, but no products will be converted to reactants.
D) A net input of energy from the surroundings is required for the reaction to proceed.
A) The products have more total energy than the reactants.
B) The reaction proceeds with a net release of free energy.
C) The reaction goes only in a forward direction: all reactants will be converted to products, but no products will be converted to reactants.
D) A net input of energy from the surroundings is required for the reaction to proceed.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following types of reactions would decrease the entropy within a cell?
A) anabolic reactions
B) hydrolysis
C) digestion
D) catabolic reactions
A) anabolic reactions
B) hydrolysis
C) digestion
D) catabolic reactions

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements best summarizes a consequence of the second law of thermodynamics?
A) If the entropy of a system increases, there must be a corresponding decrease in the entropy of the universe.
B) If the entropy of a system decreases, there must be a corresponding decrease in the entropy of the universe.
C) If entropy of a system decreases, there must be a corresponding increase in the entropy of the universe.
D) Each chemical reaction in an organism must decrease the total entropy of the universe.
A) If the entropy of a system increases, there must be a corresponding decrease in the entropy of the universe.
B) If the entropy of a system decreases, there must be a corresponding decrease in the entropy of the universe.
C) If entropy of a system decreases, there must be a corresponding increase in the entropy of the universe.
D) Each chemical reaction in an organism must decrease the total entropy of the universe.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
14
The mathematical expression for the change in free energy of a system is ΔG = ΔH - TΔS. Which of the following statements best describes a term in this equation?
A) ΔS is the change in enthalpy, a measure of randomness.
B) ΔH is the change in entropy, the energy available to do work.
C) ΔG is the change in free energy.
D) T is the temperature in degrees Celsius.
A) ΔS is the change in enthalpy, a measure of randomness.
B) ΔH is the change in entropy, the energy available to do work.
C) ΔG is the change in free energy.
D) T is the temperature in degrees Celsius.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements best describes the first law of thermodynamics?
A) Energy cannot be created or destroyed.
B) The entropy of the universe is decreasing.
C) The entropy of the universe is constant.
D) Energy cannot be transferred or transformed.
A) Energy cannot be created or destroyed.
B) The entropy of the universe is decreasing.
C) The entropy of the universe is constant.
D) Energy cannot be transferred or transformed.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following statements best summarizes an important consequence of the first law of thermodynamics for living organisms?
A) The energy content of a single organism is constant.
B) Each organism ultimately must obtain all of the necessary energy for life from its environment.
C) The entropy of an organism decreases with time as the organism grows in complexity.
D) An organism grows by converting energy into organic matter.
A) The energy content of a single organism is constant.
B) Each organism ultimately must obtain all of the necessary energy for life from its environment.
C) The entropy of an organism decreases with time as the organism grows in complexity.
D) An organism grows by converting energy into organic matter.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements best describes why hydrolysis reactions occur more readily in solution than dehydration reactions do?
A) Hydrolysis reactions increase G, or Gibbs free energy of the system.
B) Hydrolysis reactions are endergonic and increase entropy of the system.
C) Hydrolysis reactions are exergonic and decrease entropy of the system.
D) Hydrolysis reactions are exergonic and increase entropy of the system.
A) Hydrolysis reactions increase G, or Gibbs free energy of the system.
B) Hydrolysis reactions are endergonic and increase entropy of the system.
C) Hydrolysis reactions are exergonic and decrease entropy of the system.
D) Hydrolysis reactions are exergonic and increase entropy of the system.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements is the reason that most cells cannot harness heat to perform work?
A) heat is not a form of energy
B) temperature is usually uniform throughout a cell
C) heat can never be used to do work
D) heat must remain constant during work
A) heat is not a form of energy
B) temperature is usually uniform throughout a cell
C) heat can never be used to do work
D) heat must remain constant during work

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is a characteristic feature of anabolic pathways?
A) They are usually spontaneous chemical reactions.
B) They consume energy to build up polymers from monomers.
C) They release energy by degrading polymers to monomers.
D) They decrease the entropy of the organism and its environment.
A) They are usually spontaneous chemical reactions.
B) They consume energy to build up polymers from monomers.
C) They release energy by degrading polymers to monomers.
D) They decrease the entropy of the organism and its environment.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements best describes a relationship between the evolution of multicellular life on Earth and the laws of thermodynamics?
A) Evolution represents a rare exception to the second law of thermodynamics because it has resulted in such a vast diversity of complex organisms.
B) Evolution has occurred in accordance with the laws of thermodynamics and resulted in a decrease in the entropy of the universe.
C) Evolution has occurred in accordance with the laws of thermodynamics and resulted in a substantial increase in the total energy in the universe.
D) Evolution has occurred in accordance with the laws of thermodynamics and resulted in an increase in the entropy of the universe.
A) Evolution represents a rare exception to the second law of thermodynamics because it has resulted in such a vast diversity of complex organisms.
B) Evolution has occurred in accordance with the laws of thermodynamics and resulted in a decrease in the entropy of the universe.
C) Evolution has occurred in accordance with the laws of thermodynamics and resulted in a substantial increase in the total energy in the universe.
D) Evolution has occurred in accordance with the laws of thermodynamics and resulted in an increase in the entropy of the universe.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
21
When chemical, transport, or mechanical work is performed by an organism, what happens to the heat that is generated?
A) It is used to power yet more cellular work.
B) It is captured to store energy as more ATP.
C) It is used to generate ADP from nucleotide precursors.
D) It is lost to the environment.
A) It is used to power yet more cellular work.
B) It is captured to store energy as more ATP.
C) It is used to generate ADP from nucleotide precursors.
D) It is lost to the environment.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following characteristics is most likely to be associated with an enzyme that catalyzes two different chemical reactions?
A) The enzyme contains both α-helix and β-pleated sheet regions.
B) The enzyme is subject to both competitive inhibition and allosteric regulation.
C) The enzyme is composed of at least two identical subunits.
D) The enzyme has two distinct active sites.
A) The enzyme contains both α-helix and β-pleated sheet regions.
B) The enzyme is subject to both competitive inhibition and allosteric regulation.
C) The enzyme is composed of at least two identical subunits.
D) The enzyme has two distinct active sites.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
23
The ΔG of ATP hydrolysis in a test tube under standard conditions is -7.3 kcal/mol. The ΔG for the reaction A + B = C under the same conditions is +4.0 kcal/mol. What is the overall free-energy change for the coupled reactions under these conditions?
A) -11.3 kcal/mol.
B) -7.3 kcal/mol.
C) -3.3 kcal/mol.
D) +3.3 kcal/mol.
A) -11.3 kcal/mol.
B) -7.3 kcal/mol.
C) -3.3 kcal/mol.
D) +3.3 kcal/mol.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
24
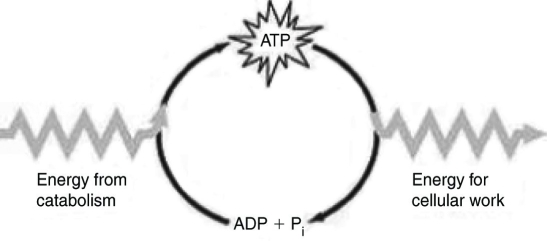 Which of the following statements best summarizes the figure above?
Which of the following statements best summarizes the figure above?A) Energy from catabolism can be used directly for performing cellular work.
B) ADP +
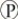 i are molecules that store energy for catabolism.
i are molecules that store energy for catabolism.C) ATP is a molecule that acts as an intermediary to store energy for cellular work.
D)
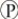 i acts as a shuttle molecule to move energy from ATP to ADP.
i acts as a shuttle molecule to move energy from ATP to ADP.
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following statements best describes catabolic pathways?
A) They combine small molecules into larger, more energy-rich molecules.
B) They require energy from ATP hydrolysis to break down polymers into monomers.
C) They are endergonic and release energy that can be used for cellular work.
D) They are exergonic and provide energy that can be used to produce ATP from ADP and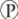 i.
i.
A) They combine small molecules into larger, more energy-rich molecules.
B) They require energy from ATP hydrolysis to break down polymers into monomers.
C) They are endergonic and release energy that can be used for cellular work.
D) They are exergonic and provide energy that can be used to produce ATP from ADP and
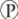 i.
i.
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
26
Catalase is an enzyme that functions in human liver cells where the pH is approximately neutral. Which of the following graphs best reflects the activity profile of catalase from pH 5 to pH 9?
A)
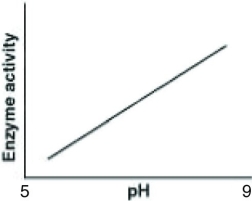
B)
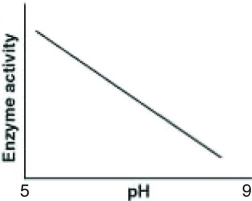
C)
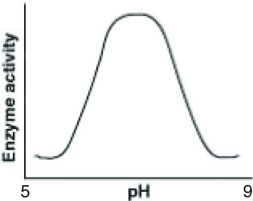
D)
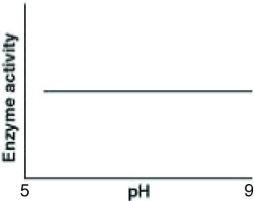
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following molecules is most similar in structure to ATP?
A) a pentose sugar
B) a DNA nucleotide
C) an RNA nucleotide
D) an amino acid with three phosphate groups attached
A) a pentose sugar
B) a DNA nucleotide
C) an RNA nucleotide
D) an amino acid with three phosphate groups attached

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements best describes induced fit?
A) Substrate binds to an allosteric site rather than to the active site of an enzyme.
B) Binding of an activator molecule changes the shape of the active site of an enzyme.
C) The conformation of the active site is determined by the tertiary or quaternary structure of the enzyme.
D) Binding of substrate to the active site changes the shape of the active site of an enzyme.
A) Substrate binds to an allosteric site rather than to the active site of an enzyme.
B) Binding of an activator molecule changes the shape of the active site of an enzyme.
C) The conformation of the active site is determined by the tertiary or quaternary structure of the enzyme.
D) Binding of substrate to the active site changes the shape of the active site of an enzyme.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
29
What is the name of the barrier that must be overcome before products are formed in a spontaneous reaction?
A) entropy
B) activation energy
C) the equilibrium point
D) free energy
A) entropy
B) activation energy
C) the equilibrium point
D) free energy

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
30
How does a noncompetitive inhibitor decrease the rate of an enzyme-catalyzed reaction?
A) by binding to the active site of the enzyme, thus preventing binding of the normal substrate
B) by binding to an allosteric site, thus changing the shape of the active site of the enzyme
C) by decreasing the free-energy change of the reaction catalyzed by the enzyme
D) by increasing the activation energy of the reaction
A) by binding to the active site of the enzyme, thus preventing binding of the normal substrate
B) by binding to an allosteric site, thus changing the shape of the active site of the enzyme
C) by decreasing the free-energy change of the reaction catalyzed by the enzyme
D) by increasing the activation energy of the reaction

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following statements best describes the central role that ATP plays in cellular metabolism?
A) Hydrolysis of ATP provides an input of free energy for exergonic reactions.
B) ATP provides energy coupling between exergonic and endergonic reactions.
C) Hydrolysis of the terminal phosphate group from ATP stores free energy that is used for cellular work.
D) The terminal phosphate bond is stronger than most covalent bonds in other biological macromolecules.
A) Hydrolysis of ATP provides an input of free energy for exergonic reactions.
B) ATP provides energy coupling between exergonic and endergonic reactions.
C) Hydrolysis of the terminal phosphate group from ATP stores free energy that is used for cellular work.
D) The terminal phosphate bond is stronger than most covalent bonds in other biological macromolecules.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
32
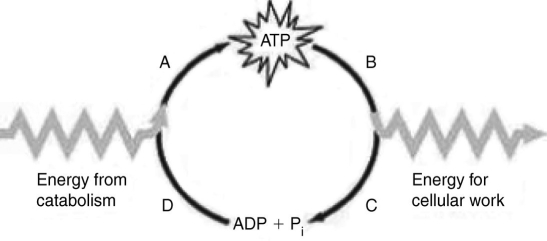 The figure presents a model of the ATP cycle in a cell. Which labeled section of the model represents the point at which ATP hydrolysis occurs?
The figure presents a model of the ATP cycle in a cell. Which labeled section of the model represents the point at which ATP hydrolysis occurs?A) A
B) B
C) C
D) D

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements best describes enzyme function?
A) Enzyme function is generally increased if the 3-D structure or conformation of an enzyme is altered.
B) Enzyme function is independent of physical and chemical environmental factors such as pH and temperature.
C) Enzymes increase the rate of chemical reactions by lowering activation energy barriers.
D) Enzymes increase the rate of chemical reactions by stimulating ATP hydrolysis.
A) Enzyme function is generally increased if the 3-D structure or conformation of an enzyme is altered.
B) Enzyme function is independent of physical and chemical environmental factors such as pH and temperature.
C) Enzymes increase the rate of chemical reactions by lowering activation energy barriers.
D) Enzymes increase the rate of chemical reactions by stimulating ATP hydrolysis.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
34
When ATP is hydrolyzed to activate a target protein, what is often the fate of the inorganic phosphate that is released?
A) It is secreted as waste.
B) It is always used to regenerate more ATP.
C) It may be used to form a phosphorylated intermediate.
D) It enters the nucleus to be incorporated in a nucleotide.
A) It is secreted as waste.
B) It is always used to regenerate more ATP.
C) It may be used to form a phosphorylated intermediate.
D) It enters the nucleus to be incorporated in a nucleotide.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
35
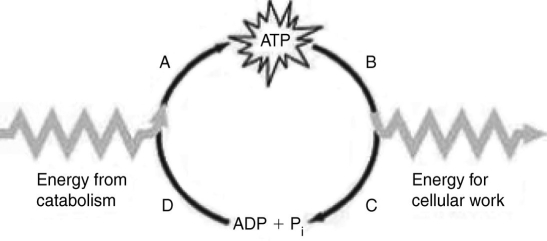 Which of the following statements best summarizes how the ATP cycle illustrated in the accompanying figure functions in cells?
Which of the following statements best summarizes how the ATP cycle illustrated in the accompanying figure functions in cells?A) The cycle is used to regenerate ATP.
B) The cycle is used to recycle energy released by ATP hydrolysis.
C) The cycle is used to provide energy for catabolism.
D) The cycle is used primarily to generate heat.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following statements best describes how addition of a catalyst will affect a chemical reaction?
A) The catalyzed reaction will have a lower ∆G than the uncatalyzed reaction.
B) The catalyzed reaction will have the same ∆G as the uncatalyzed reaction.
C) The catalyzed reaction will have a higher ∆G than the uncatalyzed reaction.
D) The catalyzed reaction will consume all of the catalyst.
A) The catalyzed reaction will have a lower ∆G than the uncatalyzed reaction.
B) The catalyzed reaction will have the same ∆G as the uncatalyzed reaction.
C) The catalyzed reaction will have a higher ∆G than the uncatalyzed reaction.
D) The catalyzed reaction will consume all of the catalyst.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following statements best describes a primary mechanism by which the energy released in ATP hydrolysis is used directly to drive endergonic chemical reactions in a cell?
A) Free energy is released as heat, which speeds up the rate of endergonic reactions.
B) The released phosphate is used to form phosphorylated intermediates that are more reactive than the original unphosphorylated substrate.
C) Binding of ATP to an enzyme active site converts an endergonic reaction to an exergonic reaction.
D) The phosphate is combined with ADP to regenerate ATP.
A) Free energy is released as heat, which speeds up the rate of endergonic reactions.
B) The released phosphate is used to form phosphorylated intermediates that are more reactive than the original unphosphorylated substrate.
C) Binding of ATP to an enzyme active site converts an endergonic reaction to an exergonic reaction.
D) The phosphate is combined with ADP to regenerate ATP.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
38
An enzyme-catalyzed reaction has a ∆G of -20 kcal/mol under standard conditions. What will be the ∆G for the reaction if the amount of enzyme is doubled?
A) -40 kcal/mol
B) -20 kcal/mol
C) -10 kcal/mol
D) +20 kcal/mol
A) -40 kcal/mol
B) -20 kcal/mol
C) -10 kcal/mol
D) +20 kcal/mol

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
39
A clasping handshake may be used as an analogy for an enzyme-catalyzed reaction because it represents the specific manner in which an enzyme ________.
A) folds to form secondary and tertiary structures
B) interacts with water
C) binds substrate
D) is denatured by low pH
A) folds to form secondary and tertiary structures
B) interacts with water
C) binds substrate
D) is denatured by low pH

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
40
The ΔG of ATP hydrolysis in a test tube under standard conditions is -7.3 kcal/mol. The ΔG for the reaction A+B C under the same conditions is +4.0 kcal/mol. How would the addition of an enzyme that catalyzes A+B C most likely alter the coupled reactions?
A) It would increase the rate of production of C and decrease the overall ΔG for the coupled reactions.
B) It would increase the rate of production of C and increase the overall ΔG for the coupled reactions.
C) It would result in no change in the rate of production of C and decrease the overall ΔG for the coupled reactions.
D) It would result in no change in the overall ΔG for the coupled reactions.
A) It would increase the rate of production of C and decrease the overall ΔG for the coupled reactions.
B) It would increase the rate of production of C and increase the overall ΔG for the coupled reactions.
C) It would result in no change in the rate of production of C and decrease the overall ΔG for the coupled reactions.
D) It would result in no change in the overall ΔG for the coupled reactions.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the terms below best describes a regulatory mechanism in which the end product of a metabolic pathway inhibits an enzyme that catalyzes an early step in the pathway?
A) allosteric inhibition
B) cooperative inhibition
C) feedback inhibition
D) metabolic inhibition
A) allosteric inhibition
B) cooperative inhibition
C) feedback inhibition
D) metabolic inhibition

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
42
Use the following information to answer the question below. 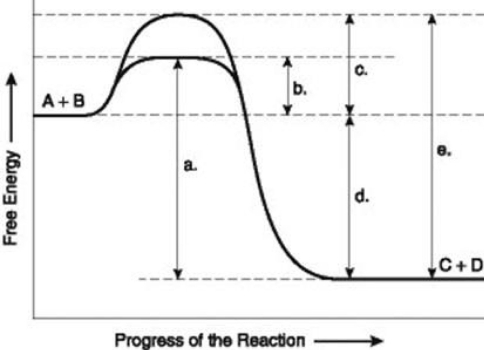 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters in the figure represents a free energy change that would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters in the figure represents a free energy change that would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?
A) a
B) b
C) c
D) d
 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters in the figure represents a free energy change that would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters in the figure represents a free energy change that would be the same in either an enzyme-catalyzed or a noncatalyzed reaction?A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
43
In the citric acid cycle, succinate dehydrogenase catalyzes the conversion of succinate to fumarate. This reaction is inhibited by malonate, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Which of the following statements best describes the role played by molecules described in the reaction?
A) Succinate dehydrogenase is the enzyme, and fumarate is the substrate in the reaction.
B) Succinate dehydrogenase is the enzyme, and malonate is the substrate in the reaction.
C) Succinate is the substrate, and fumarate is the product in the reaction.
D) Fumarate is the product, and malonate is a noncompetitive inhibitor in the reaction.
A) Succinate dehydrogenase is the enzyme, and fumarate is the substrate in the reaction.
B) Succinate dehydrogenase is the enzyme, and malonate is the substrate in the reaction.
C) Succinate is the substrate, and fumarate is the product in the reaction.
D) Fumarate is the product, and malonate is a noncompetitive inhibitor in the reaction.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
44
Students conducting research observe the rate of an enzyme-catalyzed reaction under various conditions with a fixed amount of enzyme in each sample. When will increasing the substrate concentration likely result in the greatest increase in the reaction rate?
A) when a cofactor is required in the reaction
B) when an allosteric inhibitor is present in the reaction
C) when a noncompetitive inhibitor is present in the reaction
D) when a competitive inhibitor is present in the reaction
A) when a cofactor is required in the reaction
B) when an allosteric inhibitor is present in the reaction
C) when a noncompetitive inhibitor is present in the reaction
D) when a competitive inhibitor is present in the reaction

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
45
Disruption of the active site of an enzyme would most likely result in which of the following?
A) A decreased ability of the enzyme to bind allosteric regulators.
B) An increase in the rate of the reaction catalyzed by the enzyme.
C) A decreased ability of the enzyme to bind substrates.
D) A decreased ability of the enzyme to bind products.
A) A decreased ability of the enzyme to bind allosteric regulators.
B) An increase in the rate of the reaction catalyzed by the enzyme.
C) A decreased ability of the enzyme to bind substrates.
D) A decreased ability of the enzyme to bind products.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
46
Penicillin is an antibiotic that kills bacteria by binding to the active site of an enzyme involved in synthesis of the bacterial cell wall. Which of the following phenomena best describes the mechanism of action of penicillin?
A) noncompetitive inhibition
B) feedback inhibition
C) allosteric regulation
D) competitive inhibition
A) noncompetitive inhibition
B) feedback inhibition
C) allosteric regulation
D) competitive inhibition

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
47
In the citric acid cycle, succinate dehydrogenase catalyzes the conversion of succinate to fumarate. This reaction is inhibited by malonate, which resembles succinate but cannot be acted upon by succinate dehydrogenase. Increasing the ratio of succinate molecules to those of malonate in the reaction reduces the inhibitory effect of malonate. What role does malonate play with respect to succinate dehydrogenase?
A) Malonate is a competitive inhibitor.
B) Malonate blocks the binding of fumarate.
C) Malonate is a noncompetitive inhibitor.
D) Malonate is an allosteric regulator.
A) Malonate is a competitive inhibitor.
B) Malonate blocks the binding of fumarate.
C) Malonate is a noncompetitive inhibitor.
D) Malonate is an allosteric regulator.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
48
A series of enzymes catalyze the reactions in the metabolic pathway X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme. What does substance X represent in this pathway?
A) an allosteric inhibitor
B) a substrate
C) an intermediate
D) the product
A) an allosteric inhibitor
B) a substrate
C) an intermediate
D) the product

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
49
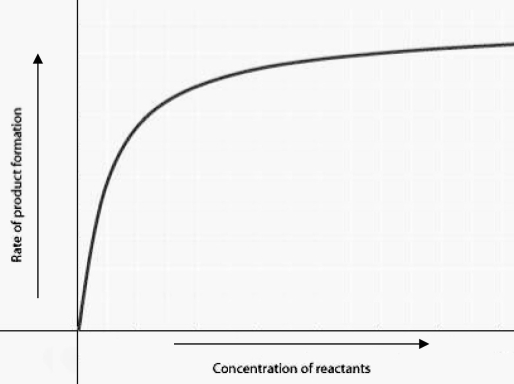 The figure displays the relationship between initial rate of product formation and reactant concentration in an enzyme-catalyzed reaction with a fixed amount of enzyme. Which of the following statements best explains the shape of the rate curve at high reactant concentration?
The figure displays the relationship between initial rate of product formation and reactant concentration in an enzyme-catalyzed reaction with a fixed amount of enzyme. Which of the following statements best explains the shape of the rate curve at high reactant concentration?A) Feedback inhibition by product occurs at high reactant concentrations.
B) Most enzyme molecules are occupied by substrate at high reactant concentrations.
C) The reaction nears equilibrium at high reactant concentrations.
D) The rate of the reverse reaction increases at high reactant concentrations.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
50
Zinc is an essential trace element for most organisms. Zinc is required in the active site of the enzyme carboxypeptidase where it most likely functions in which of the following ways?
A) as a noncompetitive inhibitor of the enzyme
B) as an allosteric activator of the enzyme
C) as a cofactor necessary for enzyme activity
D) as a coenzyme derived from a vitamin
A) as a noncompetitive inhibitor of the enzyme
B) as an allosteric activator of the enzyme
C) as a cofactor necessary for enzyme activity
D) as a coenzyme derived from a vitamin

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
51
Use the following information to answer the question below. 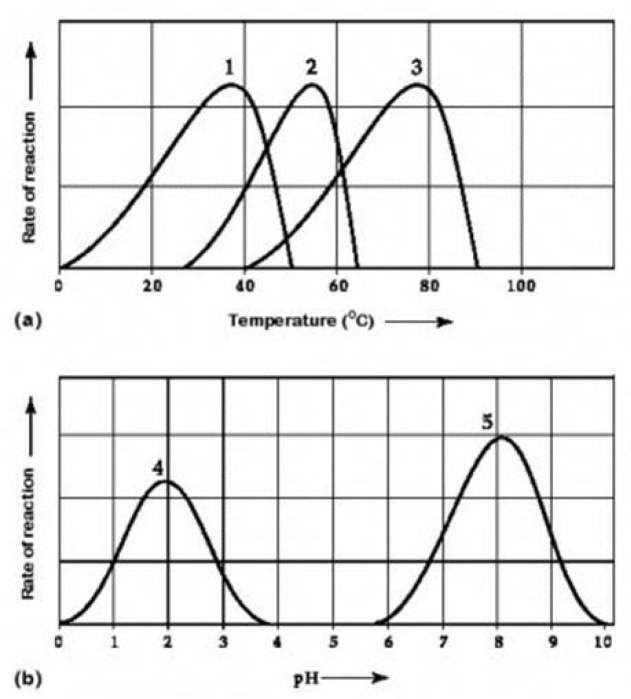 Activity of various enzymes at various temperatures (A) and at various pH (b)
Activity of various enzymes at various temperatures (A) and at various pH (b)
Which temperature and pH profile curves on the graphs are most likely associated with an enzyme isolated from a human stomach where conditions are strongly acidic?
A) curves 1 and 4
B) curves 1 and 5
C) curves 2 and 4
D) curves 3 and 4
 Activity of various enzymes at various temperatures (A) and at various pH (b)
Activity of various enzymes at various temperatures (A) and at various pH (b)Which temperature and pH profile curves on the graphs are most likely associated with an enzyme isolated from a human stomach where conditions are strongly acidic?
A) curves 1 and 4
B) curves 1 and 5
C) curves 2 and 4
D) curves 3 and 4

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
52
The 3-D structure of an enzyme composed of a single polypeptide chain includes a large substrate-binding domain. It also has a separate binding site for a regulatory molecule. Based on these structural observations the enzyme is most likely regulated by which of the following mechanism?
A) noncompetitive inhibition
B) competitive activation
C) competitive inhibition
D) cooperativity
A) noncompetitive inhibition
B) competitive activation
C) competitive inhibition
D) cooperativity

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
53
In addition to activating or inhibiting enzymes through allosteric regulation, which of these mechanisms do cells also use to control enzymatic activity?
A) localization of enzymes into specific organelles or membranes
B) secretion of enzymes out of the cell
C) assembly of enzymes into large aggregates
D) altering internal pH
A) localization of enzymes into specific organelles or membranes
B) secretion of enzymes out of the cell
C) assembly of enzymes into large aggregates
D) altering internal pH

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
54
A series of enzymes catalyze the reactions in the metabolic pathway X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme. Based on its interaction with the enzyme that converts X to Y substance A is best described as ________.
A) an allosteric inhibitor
B) a substrate
C) a metabolic intermediate
D) a competitive inhibitor
A) an allosteric inhibitor
B) a substrate
C) a metabolic intermediate
D) a competitive inhibitor

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
55
A change of a single amino acid at a position distant from the active site of an enzyme can alter the substrate specificity by changing the ________.
A) by changing the intracellular location of the enzyme
B) by changing the 3-D shape of the enzyme
C) by changing the stability the enzyme
D) by changing the binding site for a competitive inhibitor
A) by changing the intracellular location of the enzyme
B) by changing the 3-D shape of the enzyme
C) by changing the stability the enzyme
D) by changing the binding site for a competitive inhibitor

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
56
Use the following information to answer the question below. 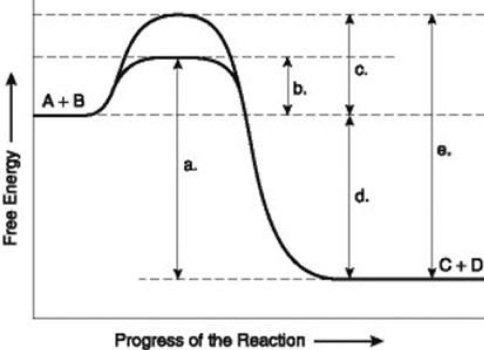 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describe the forward reaction in the figure?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describe the forward reaction in the figure?
A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0
 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describe the forward reaction in the figure?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describe the forward reaction in the figure?A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
57
Use the following information to answer the question below. 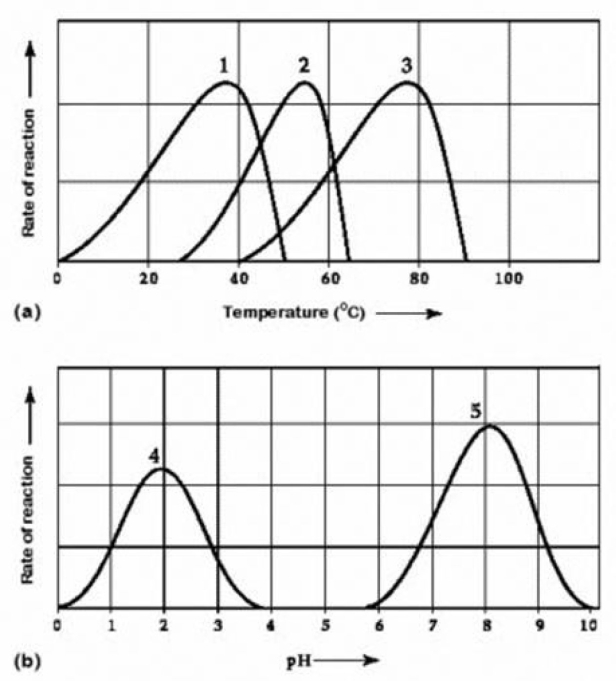 Activity of various enzymes at various temperatures (a) and at various pH (b)
Activity of various enzymes at various temperatures (a) and at various pH (b)
Which curves on the graphs most likely represent the temperature and pH profiles of an enzyme taken from a bacterium that lives in a mildly alkaline (basic) hot springs at temperatures of 70°C or higher?
A) curves 1 and 5
B) curves 2 and 5
C) curves 3 and 4
D) curves 3 and 5
 Activity of various enzymes at various temperatures (a) and at various pH (b)
Activity of various enzymes at various temperatures (a) and at various pH (b)Which curves on the graphs most likely represent the temperature and pH profiles of an enzyme taken from a bacterium that lives in a mildly alkaline (basic) hot springs at temperatures of 70°C or higher?
A) curves 1 and 5
B) curves 2 and 5
C) curves 3 and 4
D) curves 3 and 5

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
58
Use the following information to answer the question below. 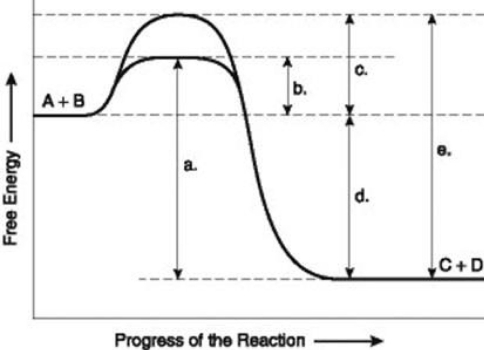 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters represents the activation energy required for the enzyme-catalyzed reaction in the figure?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters represents the activation energy required for the enzyme-catalyzed reaction in the figure?
A) a
B) b
C) c
D) d
 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters represents the activation energy required for the enzyme-catalyzed reaction in the figure?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters represents the activation energy required for the enzyme-catalyzed reaction in the figure?A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
59
Use the following information to answer the question below. 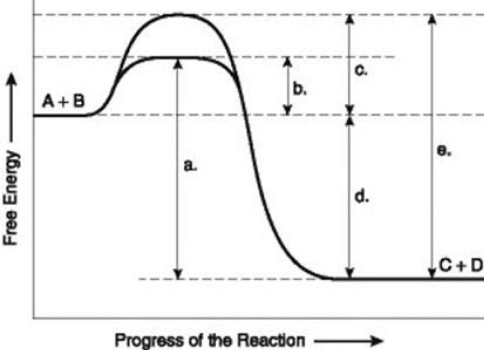 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters represents the activation energy required for the non-enzyme-catalyzed reaction in the figure?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters represents the activation energy required for the non-enzyme-catalyzed reaction in the figure?
A) a
B) b
C) c
D) d
 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters represents the activation energy required for the non-enzyme-catalyzed reaction in the figure?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the lower-case letters represents the activation energy required for the non-enzyme-catalyzed reaction in the figure?A) a
B) b
C) c
D) d

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
60
An enzyme is composed of four identical subunits. Binding of substrate to one subunit stimulates more rapid binding of substrate to each of the other three subunits. This enzyme is most likely regulated by which of the following mechanisms?
A) noncompetitive inhibition
B) competitive activation
C) competitive inhibition
D) cooperativity
A) noncompetitive inhibition
B) competitive activation
C) competitive inhibition
D) cooperativity

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
61
Choose the pair of terms that correctly completes this sentence: Catabolism is to anabolism as ________ is to ________.
A) exergonic; spontaneous
B) exergonic; endergonic
C) free energy; entropy
D) work; energy
A) exergonic; spontaneous
B) exergonic; endergonic
C) free energy; entropy
D) work; energy

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
62
If an enzyme in solution is saturated with substrate, the most effective way to obtain a faster yield of products is to ________.
A) add more of the enzyme
B) heat the solution to 90°C
C) add more substrate
D) add a noncompetitive inhibitor
A) add more of the enzyme
B) heat the solution to 90°C
C) add more substrate
D) add a noncompetitive inhibitor

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
63
If an enzyme is added to a solution where its substrate and product are in equilibrium, what will occur?
A) Additional substrate will be formed.
B) The reaction will change from endergonic to exergonic.
C) The free energy of the system will change.
D) Nothing; the reaction will stay at equilibrium.
A) Additional substrate will be formed.
B) The reaction will change from endergonic to exergonic.
C) The free energy of the system will change.
D) Nothing; the reaction will stay at equilibrium.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
64
Most cells cannot harness heat to perform work because
A) heat does not involve a transfer of energy.
B) cells do not have much thermal energy; they are relatively cool.
C) temperature is usually uniform throughout a cell.
D) heat can never be used to do work.
A) heat does not involve a transfer of energy.
B) cells do not have much thermal energy; they are relatively cool.
C) temperature is usually uniform throughout a cell.
D) heat can never be used to do work.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
65
Some bacteria are metabolically active in hot springs because ________.
A) they are able to maintain a lower internal temperature
B) high temperatures make catalysis unnecessary
C) their enzymes have high optimal temperatures
D) their enzymes are completely insensitive to temperature
A) they are able to maintain a lower internal temperature
B) high temperatures make catalysis unnecessary
C) their enzymes have high optimal temperatures
D) their enzymes are completely insensitive to temperature

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck


