Deck 9: Thermal Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 9: Thermal Physics
1
An athlete doing push-ups performs 700 kJ of work and loses 455 kJ of heat. What is the change in the internal energy of the athlete?
A)1155 kJ
B)245 kJ
C)-245 kJ
D)-1155 kJ
A)1155 kJ
B)245 kJ
C)-245 kJ
D)-1155 kJ
-1155 kJ
2
How is the molar heat capacity C of a substance of molar mass M related to the specific heat capacity c?
A)C = nc, where n = number of moles
B)c = nC, where n = number of moles
C)c = MC
D)C = Mc
A)C = nc, where n = number of moles
B)c = nC, where n = number of moles
C)c = MC
D)C = Mc
C = Mc
3
An iron bullet of mass 100 g and travelling at 500 m/s hits a fixed iron plate of mass 400G) Assume the bullet is brought to rest on hitting the plate.What temperature rise occurs in the iron, assuming the temperature distribution is uniform?
A)70°C
B)56°C
C)7°C
D)6°C
A)70°C
B)56°C
C)7°C
D)6°C
56°C
4
In comparing adiabatic and isothermal compressions of a gas from the same initial pressure and volume, in which one of the following ways do you expect the pressure to behave differently between the two processes?
A)The pressures remain equal to each other during compression.
B)The pressure climbs more rapidly for adiabatic compression.
C)More heat is available to raise the pressure in adiabatic compression than in isothermal compression.
D)The pressure climbs more rapidly for isothermal compression.
A)The pressures remain equal to each other during compression.
B)The pressure climbs more rapidly for adiabatic compression.
C)More heat is available to raise the pressure in adiabatic compression than in isothermal compression.
D)The pressure climbs more rapidly for isothermal compression.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
What happens during an isochoric process when the temperature of a gas is increased by a factor of 10%?
A)The volume increases by 10%.
B)The pressure increases by 10%.
C)Both volume and pressure increase.
D)Pressure increases by less than 10%, with the balance contributing to expansion of the gas.
A)The volume increases by 10%.
B)The pressure increases by 10%.
C)Both volume and pressure increase.
D)Pressure increases by less than 10%, with the balance contributing to expansion of the gas.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
An athlete doing push-ups performs 700 kJ of work and loses 450 kJ of heat. What is the change in the internal energy of the athlete?
A)1150 kJ
B)250 kJ
C)-250 kJ
D)-1150 kJ
A)1150 kJ
B)250 kJ
C)-250 kJ
D)-1150 kJ

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
An ideal monatomic gas expands at a constant pressure of 120 kPa from an initial volume of 2.0 L to a final volume of 5.0 L. If the temperature of the gas remains constant, how much heat flows into the gas?
A)360 J
B)-360 J
C)3.6 kJ
D)-3 kJ
A)360 J
B)-360 J
C)3.6 kJ
D)-3 kJ

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
Figure 9.1 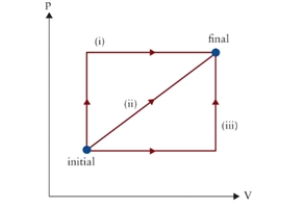 p-V diagram of several possible thermodynamic processes between initial and final states.
p-V diagram of several possible thermodynamic processes between initial and final states.
In Fig. 9.1, by which closed path, starting and ending at "initial" and passing through "final," will the most work be done on the gas?
A)(iii) followed by reverse of (i)
B)(iii) followed by reverse of (ii)
C)(i) followed by reverse of (iii)
D)(ii) followed by reverse of (i)
 p-V diagram of several possible thermodynamic processes between initial and final states.
p-V diagram of several possible thermodynamic processes between initial and final states.In Fig. 9.1, by which closed path, starting and ending at "initial" and passing through "final," will the most work be done on the gas?
A)(iii) followed by reverse of (i)
B)(iii) followed by reverse of (ii)
C)(i) followed by reverse of (iii)
D)(ii) followed by reverse of (i)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
A monatomic ideal gas with an initial pressure of 100 kPa and an initial volume of 1.50 L expands isothermally to a final volume of 5.00 L. How much work is done on the gas in this process?
A)181 J
B)78 J
C)-78 J
D)-181 J
A)181 J
B)78 J
C)-78 J
D)-181 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
An expandable container holds 2.50 moles of He gas with an initial pressure of 750 kPa and an initial volume of 2.0 L. The gas expands isothermally to a final pressure of 375 kPa. How much heat is gained by the gas in this process?
A)1040 J
B)452 J
C)-452 J
D)-1040 J
A)1040 J
B)452 J
C)-452 J
D)-1040 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
A gas expands from an initial volume of 0.015 m3 to a final volume of 0.075 m3, while its pressure increases linearly with the volume (so that the process follows a straight-line path in a p-V diagram) from 80 kPa to 275 kPa. How much work is done by the system?
A)-10.6 kJ
B)-5.6 kJ
C)5.6 kJ
D)10.6 kJ
A)-10.6 kJ
B)-5.6 kJ
C)5.6 kJ
D)10.6 kJ

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
2.0 moles of an ideal monatomic gas are initially at a temperature of 300 K. If the gas gains 2800 J of heat and performs 400 J of work, what is its final temperature?
A)515 K
B)396 K
C)251 K
D)204 K
A)515 K
B)396 K
C)251 K
D)204 K

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
Figure 9.1  p-V diagram of several possible thermodynamic processes between initial and final states.
p-V diagram of several possible thermodynamic processes between initial and final states.
A fixed mass of gas initially occupies a 2 L volume at atmospheric pressure. It undergoes an isobaric change to a volume of 5 L, followed by an isochoric change to a pressure of 2 atm. See path (iii) in Fig. 9.1. If the system is brought back to its initial state along the reverse of path (ii), what is the net work done on the gas?
A)-456 J
B)-152 J
C)152 J
D)456 J
 p-V diagram of several possible thermodynamic processes between initial and final states.
p-V diagram of several possible thermodynamic processes between initial and final states.A fixed mass of gas initially occupies a 2 L volume at atmospheric pressure. It undergoes an isobaric change to a volume of 5 L, followed by an isochoric change to a pressure of 2 atm. See path (iii) in Fig. 9.1. If the system is brought back to its initial state along the reverse of path (ii), what is the net work done on the gas?
A)-456 J
B)-152 J
C)152 J
D)456 J

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements best describes the characteristics of gauge pressure?
A)Gauge pressure is always positive.
B)When absolute pressure is lower than atmospheric pressure, the gauge pressure is positive.
C)Gauge pressure depends on atmospheric pressure.
D)Positive gauge pressure gives a system the propensity to expand.
A)Gauge pressure is always positive.
B)When absolute pressure is lower than atmospheric pressure, the gauge pressure is positive.
C)Gauge pressure depends on atmospheric pressure.
D)Positive gauge pressure gives a system the propensity to expand.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
A gas expands from an initial volume of 5.0 L to a final volume of 20.0 L at a constant pressure of 350 kPa. How much work is done by the gas?
A)8.75 kJ
B)5.25 kJ
C)-5.25 kJ
D)-8.75 kJ
A)8.75 kJ
B)5.25 kJ
C)-5.25 kJ
D)-8.75 kJ

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
In which one of these ways does the pressure in lungs at rest compare with atmospheric pressure?
A)always higher
B)always lower
C)always the same
D)varies during the breathing cycle
A)always higher
B)always lower
C)always the same
D)varies during the breathing cycle

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
A gas is heated at a constant pressure of 135 kPa, from an initial volume of 0.040 m3 and temperature of 20°C to a final volume of 0.06 m3 and a temperature of 25°C. How much heat flows into or out of the system?
A)1.3 kJ into system
B)2.6 kJ out of system
C)2.6 kJ into system
D)5.2 kJ out of system
A)1.3 kJ into system
B)2.6 kJ out of system
C)2.6 kJ into system
D)5.2 kJ out of system

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
How does the molar heat capacity of an ideal gas relate to the composition of that gas?
A)It is constant for all gases.
B)It depends on the number of moles of gas.
C)It depends on the mass of gas.
D)It varies with temperature.
A)It is constant for all gases.
B)It depends on the number of moles of gas.
C)It depends on the mass of gas.
D)It varies with temperature.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
Figure 9.2 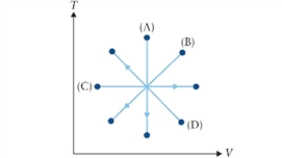 T-V diagram with four processes for an ideal gas.
T-V diagram with four processes for an ideal gas.
In Fig. 9.2, which process does path (B) represent?
A)isobaric expansion
B)isochoric compression
C)isobaric compression
D)isothermal compression
 T-V diagram with four processes for an ideal gas.
T-V diagram with four processes for an ideal gas.In Fig. 9.2, which process does path (B) represent?
A)isobaric expansion
B)isochoric compression
C)isobaric compression
D)isothermal compression

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
In Joule's experiment, if a 10.0 kg mass falls a distance of 1.0 m, and the paddle is immersed in a 0.5 L beaker of mercury, what is the rise in temperature of the mercury, given that its mass density is 13.5 g/cm3 and its specific heat capacity is 138 J/(kg °C)?
A)1.0°C
B)0.1°C
C)0.01°C
D)0.001°C
A)1.0°C
B)0.1°C
C)0.01°C
D)0.001°C

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
If the fluid in Joule's experiment were replaced by an equal mass of mercury, the measured rise in temperature would be greater than that for water.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
Under which of these conditions does an ideal gas experience the greatest gain in internal energy while undergoing reversible compression?
A)at constant pressure
B)at constant temperature
C)adiabatically
D)when pressure is proportional to volume
A)at constant pressure
B)at constant temperature
C)adiabatically
D)when pressure is proportional to volume

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
In normal breathing, the majority of the work required comes from the inhaled air.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following happens when a gas expands adiabatically?
A)The internal energy increases.
B)The internal energy decreases.
C)There is no work done by the gas.
D)There is work done on the gas.
A)The internal energy increases.
B)The internal energy decreases.
C)There is no work done by the gas.
D)There is work done on the gas.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
An ideal monatomic gas undergoes a reversible expansion to twice its original volume. Under which one of the following conditions does the gas perform the least amount of work?
A)at constant pressure
B)at constant temperature
C)adiabatically
D)as pressure increases in proportion to its volume
A)at constant pressure
B)at constant temperature
C)adiabatically
D)as pressure increases in proportion to its volume

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
For an ideal gas undergoing an isochoric process, the rise in temperature is proportional to the amount of heat absorbed.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
During inhalation, the pressure in the lungs is less than atmospheric pressure.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
Which of these statements does NOT describe a reversible process?
A)The system can be returned to its original state.
B)The system passes through a continuous sequence of equilibrium states.
C)Behaviour of the system is the same when going forward in time as when going backward.
D)The system and its environment can be returned to their original states.
A)The system can be returned to its original state.
B)The system passes through a continuous sequence of equilibrium states.
C)Behaviour of the system is the same when going forward in time as when going backward.
D)The system and its environment can be returned to their original states.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
Entropy is a variable of state.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
A diesel engine uses adiabatic heating to ignite the fuel.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
Compressing gas in a closed system does negative work on the gas.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
In a reversible process, the temperature of an ideal gas is kept constant as the gas is compressed. The gas must absorb heat from its surroundings.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
When a gas undergoes an isothermal expansion, its entropy increases.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
Cyclic processes may be reversible or irreversible.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
An ideal monatomic gas undergoes a reversible expansion to twice its original volume. Under which of these conditions does the gas perform the most work?
A)at constant pressure
B)at constant temperature
C)adiabatically
D)pressure increases in proportion to its volume
A)at constant pressure
B)at constant temperature
C)adiabatically
D)pressure increases in proportion to its volume

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
In the first law of thermodynamics, W is the work done on the system; that is, W is positive if work is done on the system.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
A certain ideal gas has an adiabatic coefficient of ê = 4/3. What is its molar specific heat at constant pressure?
A)4R
B)5R/3
C)3R/2
D)2R/3
A)4R
B)5R/3
C)3R/2
D)2R/3

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
Which of these statements accurately describes how the efficiency of the Carnot cycle depends on the system?
A)It depends on the amount of heat absorbed or lost during each isothermal phase.
B)It is a function of the initial and final temperatures of the heat reservoirs.
C)It is different when the cycle is performed in reverse.
D)It is independent of any system parameters.
A)It depends on the amount of heat absorbed or lost during each isothermal phase.
B)It is a function of the initial and final temperatures of the heat reservoirs.
C)It is different when the cycle is performed in reverse.
D)It is independent of any system parameters.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
At what lower temperature does a reversible engine operating from a high temperature of 600 K have the same efficiency as another reversible engine operating between 400 K and 750 K?
A)350 K
B)330 K
C)320 K
D)290 K
A)350 K
B)330 K
C)320 K
D)290 K

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
The sum of all energy forms in a closed system is constant.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
For a polyatomic gas, some internal energy may be stored as vibrational and/or rotational energy within the molecules. Describe what effect this will have on the adiabatic coefficient ê, which has the value 5/3 when only translational motion of molecules is allowed.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
When a parcel of air is heated over a surface, it expands, thereby lowering its density relative to its surroundings, and experiences a buoyancy force, causing it to rise. Suppose that the ambient air is cold at the bottom and warmer at the top, giving rise to a "thermal inversion." What do you expect would happen to the heated parcel of air if this inversion were sufficiently strong, assuming the parcel expands adiabatically?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
Explain the difference between gauge pressure and absolute pressure.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
What characterizes the thermal energy of a system? Explain the difference between heat and thermal energy.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
Describe what distinguishes thermal energy from other forms of energy.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
Two identical samples of gas at the same pressure and volume are compressed to the same final volume. If the first sample undergoes isothermal compression and the second sample adiabatic compression, compare the outcomes of the two processes.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
Entropy of isolated systems undergoing irreversible processes increases and reaches a maximum when equilibrium is reached. What implications does this have for our universe?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
How did Joule's experiments lead to the formulation of the first law of thermodynamics?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
Describe how you might use a waterfall to measure the equivalence of thermal and mechanical heat. What complications might make this experiment difficult to perform?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
Describe how you would improve the efficiency of a real engine operating between two reservoirs at temperatures Thigh and Tlow.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
In the Carnot process, described in Section 9.6.2, heat is absorbed from a reservoir at a high temperature and heat is released to another reservoir at a lower temperature. Is it possible to run the cycle in reverse? If so, what effect would be achieved?

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
Given some experimental data on pressure-versus-volume measurements, describe, in practical terms, how you would determine the work done by or on a system undergoing a cyclic process.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


