Deck 13: Alcohols, Phenols, Thiols, and Ethers
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/49
Play
Full screen (f)
Deck 13: Alcohols, Phenols, Thiols, and Ethers
1
Which one of the following compounds is an alcohol?
A)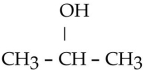
B)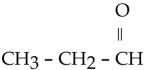
C)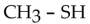
D)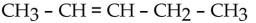
E)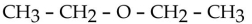
A)
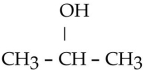
B)
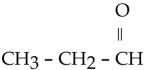
C)
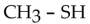
D)
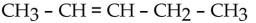
E)
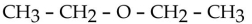
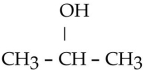
2
The common name for the compound 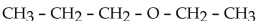 is
is
A)3-ether pentane.
B)3-pentanol.
C)3-hexanol.
D)ethyl propyl ketone.
E)ethyl propyl ether.
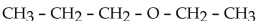 is
isA)3-ether pentane.
B)3-pentanol.
C)3-hexanol.
D)ethyl propyl ketone.
E)ethyl propyl ether.
ethyl propyl ether.
3
Alcohols contain which functional group?
A)amide
B)amine
C)hydroxyl
D)thiol
A)amide
B)amine
C)hydroxyl
D)thiol
hydroxyl
4
What is the common name of this compound? 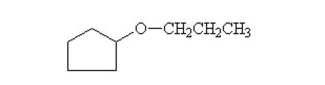
A)propylcyclopentanol
B)cyclopentyl propyl ketone
C)3-cyclopentylpropanol
D)cyclopentyl propyl ether
E)1-cyclopropyl-1-propylalcohol

A)propylcyclopentanol
B)cyclopentyl propyl ketone
C)3-cyclopentylpropanol
D)cyclopentyl propyl ether
E)1-cyclopropyl-1-propylalcohol

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
5
What is the IUPAC name for 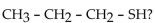
A)2-propanethiol
B)propyl thiol
C)2-butanethiol
D)1-propanethiol
E)1-butanethiol
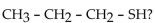
A)2-propanethiol
B)propyl thiol
C)2-butanethiol
D)1-propanethiol
E)1-butanethiol

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
6
Why has diethyl ether been replaced as a general anesthetic?
A)It is slightly polar.
B)It can hydrogen bond to water.
C)It is very volatile and highly flammable.
D)It is slightly soluble in water.
E)It is not very reactive.
A)It is slightly polar.
B)It can hydrogen bond to water.
C)It is very volatile and highly flammable.
D)It is slightly soluble in water.
E)It is not very reactive.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following compounds is a thiol?
A)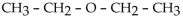
B)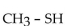
C)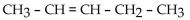
D)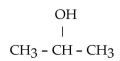
E)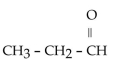
A)
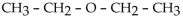
B)
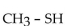
C)
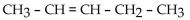
D)
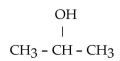
E)
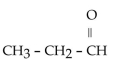

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following does NOT involve methanol?
A)alcoholic beverages
B)paint remover
C)solvents
D)making plastics
E)racing fuel
A)alcoholic beverages
B)paint remover
C)solvents
D)making plastics
E)racing fuel

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
9
What is the name for this compound?
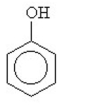
A) cyclobenzenol
B) cyclohexanol
C) cyclopentanol
D) glycerol
E) phenol

A) cyclobenzenol
B) cyclohexanol
C) cyclopentanol
D) glycerol
E) phenol

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
10
What is the IUPAC name for this compound?
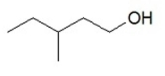
A) 3-methyl-5-pentanol
B) 3-methylpentanol
C) 3-methyl-1-pentanol
D) 3-methyl-6-hexanol
E) 3-methyl-1-hexanol

A) 3-methyl-5-pentanol
B) 3-methylpentanol
C) 3-methyl-1-pentanol
D) 3-methyl-6-hexanol
E) 3-methyl-1-hexanol

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
11
Thiols are strong-smelling compounds responsible for
A)salty odors.
B)flowery odors.
C)fruity odors.
D)sharp odors.
E)skunky or bad-smelling odors.
A)salty odors.
B)flowery odors.
C)fruity odors.
D)sharp odors.
E)skunky or bad-smelling odors.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the IUPAC name for the compound below? 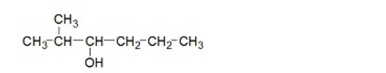
A)2-methyl-3-heptanol
B)5-methyl-4-hexanol
C)5-methyl-4-pentanol
D)2-methyl-3-hexanol
E)2-methyl-3-pentanol

A)2-methyl-3-heptanol
B)5-methyl-4-hexanol
C)5-methyl-4-pentanol
D)2-methyl-3-hexanol
E)2-methyl-3-pentanol

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
13
The condensed structural formula for 2,3-dichloro-4-methylcyclohexanol is
A)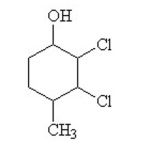
B)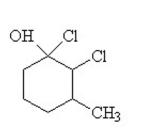
C)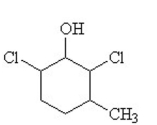
D)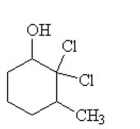
E)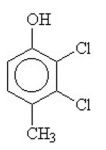
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
14
1,2-ethanediol (ethylene glycol)has uses that include
A)production of synthetic fibers.
B)solvent for paint.
C)solvent for ink.
D)antifreeze.
E)All of the above.
A)production of synthetic fibers.
B)solvent for paint.
C)solvent for ink.
D)antifreeze.
E)All of the above.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name of this compound? 
A)2-propanol
B)2-methyl-2-propanol
C)butanol
D)propanol
E)2-methylbutanol

A)2-propanol
B)2-methyl-2-propanol
C)butanol
D)propanol
E)2-methylbutanol

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
16
What is the name for this compound? 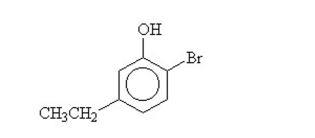
A)4-bromo-1-ethyl-5-phenol
B)6-bromo-3-ethylphenol
C)2-bromo-5-methylphenol
D)2-bromo-5-ethylcyclohexanol
E)2-bromo-5-ethylphenol

A)4-bromo-1-ethyl-5-phenol
B)6-bromo-3-ethylphenol
C)2-bromo-5-methylphenol
D)2-bromo-5-ethylcyclohexanol
E)2-bromo-5-ethylphenol

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
17
The compound 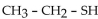 is in the organic family known as
is in the organic family known as
A) ethers.
B) sulfides.
C) amino acids.
D) alcohols.
E) thiols.
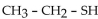 is in the organic family known as
is in the organic family known asA) ethers.
B) sulfides.
C) amino acids.
D) alcohols.
E) thiols.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
18
The common name of CH3-CH2-O-CH2-CH3 is
A)butyl ether.
B)dimethyl ether.
C)dibutyl ether.
D)diethyl ether.
E)2-etherbutane.
A)butyl ether.
B)dimethyl ether.
C)dibutyl ether.
D)diethyl ether.
E)2-etherbutane.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
19
A phenol has an -OH group bonded to a(n)
A)disubstituted carbon.
B)singly substituted or unsubstituted carbon.
C)tetrasubstituted carbon.
D)aromatic carbon.
E)trisubstituted carbon.
A)disubstituted carbon.
B)singly substituted or unsubstituted carbon.
C)tetrasubstituted carbon.
D)aromatic carbon.
E)trisubstituted carbon.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
20
Thiols have structures similar to alcohols except that they contain
A)more than one carbon.
B)nitrogen in place of oxygen in the functional group.
C)sulfur in place of oxygen in the functional group.
D)lithium in place of oxygen in the functional group.
E)three alcohol groups.
A)more than one carbon.
B)nitrogen in place of oxygen in the functional group.
C)sulfur in place of oxygen in the functional group.
D)lithium in place of oxygen in the functional group.
E)three alcohol groups.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
21
What type of alcohol is resistant to oxidation?
A)primary
B)secondary
C)tertiary
D)quaternary
E)none
A)primary
B)secondary
C)tertiary
D)quaternary
E)none

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
22
In a tertiary alcohol, how many alkyl groups are attached to the carbon atom bonded to the -OH group?
A)none
B)one
C)two
D)three
E)four
A)none
B)one
C)two
D)three
E)four

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
23
A secondary alcohol has a hydroxyl group bonded to a(n)
A)carbon that is bonded to two alkyl groups.
B)aromatic carbon.
C)carbon that is bonded to one alkyl group.
D)carbon that is bonded to three alkyl groups.
A)carbon that is bonded to two alkyl groups.
B)aromatic carbon.
C)carbon that is bonded to one alkyl group.
D)carbon that is bonded to three alkyl groups.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
24
The dehydration product of 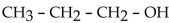 in the presence of acid is
in the presence of acid is
A) cyclopropene.
B) propene.
C) cyclopropane.
D)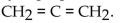
E) propyne.
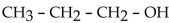 in the presence of acid is
in the presence of acid isA) cyclopropene.
B) propene.
C) cyclopropane.
D)
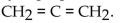
E) propyne.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following compounds is a secondary alcohol?
A)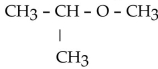
B)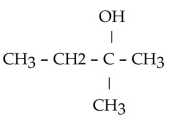
C)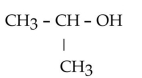
D)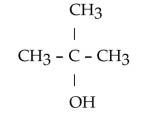
E)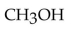
A)
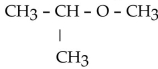
B)

C)
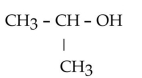
D)

E)
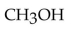

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
26
What is the product when this compound undergoes oxidation? 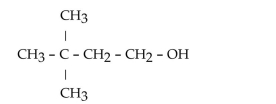
A)3,3-dimethylbutanal
B)hexanal
C)2,2-dimethylbutanal
D)3,3-dimethyl-1-butanone
E)2,2-dimethyl-4-butanone
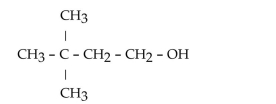
A)3,3-dimethylbutanal
B)hexanal
C)2,2-dimethylbutanal
D)3,3-dimethyl-1-butanone
E)2,2-dimethyl-4-butanone

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
27
A tertiary alcohol has a hydroxyl group bonded to a
A)carbon that is bonded to three alkyl groups.
B)double bonded carbon.
C)carbon that is bonded to one alkyl group.
D)triple-bonded carbon.
E)carbon that is bonded to two alkyl groups.
A)carbon that is bonded to three alkyl groups.
B)double bonded carbon.
C)carbon that is bonded to one alkyl group.
D)triple-bonded carbon.
E)carbon that is bonded to two alkyl groups.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
28
The IUPAC name for the compound CH3-CH2-CH2-O-CH2-CH3 is
A)2-ethoxyethane.
B)1-propoxyethane.
C)ethyl propyl ether.
D)1-ethoxypropane.
E)2-ethoxypropane.
A)2-ethoxyethane.
B)1-propoxyethane.
C)ethyl propyl ether.
D)1-ethoxypropane.
E)2-ethoxypropane.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
29
What is the product when the following compound is oxidized? 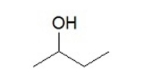
A)an aldehyde
B)an ether
C)an alkane
D)an alkene
E)a ketone

A)an aldehyde
B)an ether
C)an alkane
D)an alkene
E)a ketone

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following compounds is a weak acid?
A)cyclohexanol
B)phenol
C)ethanal
D)ethanol
E)acetone
A)cyclohexanol
B)phenol
C)ethanal
D)ethanol
E)acetone

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
31
The alcohol in this list that would be most soluble in water is
A)1-heptanol.
B)ethanol.
C)1-butanol.
D)1-pentanol.
E)1-hexanol.
A)1-heptanol.
B)ethanol.
C)1-butanol.
D)1-pentanol.
E)1-hexanol.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
32
Tertiary alcohols cannot be oxidized because
A)the alcohol carbon is bonded to four groups so no hydrogen can be added to it.
B)the alcohol carbon is bonded to four groups so no oxygen can be added to it.
C)there are no oxygen atoms to remove from the alcohol carbon.
D)there are no hydrogen atoms attached to the alcohol carbon.
E)the alcohol carbon is too electronegative to have hydrogen removed from it.
A)the alcohol carbon is bonded to four groups so no hydrogen can be added to it.
B)the alcohol carbon is bonded to four groups so no oxygen can be added to it.
C)there are no oxygen atoms to remove from the alcohol carbon.
D)there are no hydrogen atoms attached to the alcohol carbon.
E)the alcohol carbon is too electronegative to have hydrogen removed from it.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
33
What kind of bonds do alcohols form between individual molecules?
A)carbon bonds
B)hydrogen bonds
C)oxygen bonds
D)single bonds
E)ionic bonds
A)carbon bonds
B)hydrogen bonds
C)oxygen bonds
D)single bonds
E)ionic bonds

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
34
What is the common name of the ether that is an isomer of 2-propanol?
A)isopropyl ether
B)ethyl methyl ether
C)1-propanol
D)dimethyl ether
E)diethyl ether
A)isopropyl ether
B)ethyl methyl ether
C)1-propanol
D)dimethyl ether
E)diethyl ether

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
35
A primary alcohol has a hydroxyl group bonded to a(n)
A)aromatic carbon.
B)carbon that is bonded to one alkyl group.
C)carbon that is bonded to two alkyl groups.
D)carbon that is bonded to three alkyl groups.
A)aromatic carbon.
B)carbon that is bonded to one alkyl group.
C)carbon that is bonded to two alkyl groups.
D)carbon that is bonded to three alkyl groups.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
36
What type of alcohol undergoes oxidation to yield a ketone?
A)primary alcohol
B)secondary alcohol
C)both primary and secondary alcohols
D)all classes of alcohols
E)both secondary and tertiary alcohols
A)primary alcohol
B)secondary alcohol
C)both primary and secondary alcohols
D)all classes of alcohols
E)both secondary and tertiary alcohols

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
37
What is the common name of the ether that is an isomer of 1-butanol?
A)butyl ether
B)dimethyl ether
C)isopropyl ether
D)diethyl ether
E)ethyl methyl ether
A)butyl ether
B)dimethyl ether
C)isopropyl ether
D)diethyl ether
E)ethyl methyl ether

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
38
The dehydration of an alcohol in the presence of a strong acid yields
A)an alkane.
B)a ketone.
C)an alkene.
D)an alcohol.
E)an aldehyde.
A)an alkane.
B)a ketone.
C)an alkene.
D)an alcohol.
E)an aldehyde.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
39
When 2-methyl-2-butanol undergoes dehydration in acid, the major product is
A)2-methylbutanal.
B)2-methyl-2-butene.
C)2-methylbutanone.
D)2-pentanone.
E)hexene.
A)2-methylbutanal.
B)2-methyl-2-butene.
C)2-methylbutanone.
D)2-pentanone.
E)hexene.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
40
In the oxidation of an alcohol to a ketone, there is
A)a loss of carbon.
B)a loss of hydrogen.
C)a gain of oxygen.
D)a loss of oxygen.
E)a gain of hydrogen.
A)a loss of carbon.
B)a loss of hydrogen.
C)a gain of oxygen.
D)a loss of oxygen.
E)a gain of hydrogen.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
41
Which compound will undergo oxidation to yield the following?
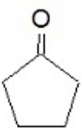
A)pentanol
B)methylcyclobutanol
C)cyclopentene
D)cyclopentanol
E)cyclopentane

A)pentanol
B)methylcyclobutanol
C)cyclopentene
D)cyclopentanol
E)cyclopentane

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
42
Alcohols undergo combustion to form
A)aldehydes.
B)alkenes.
C)water, carbon dioxide, and energy.
D)ethers.
E)ketones.
A)aldehydes.
B)alkenes.
C)water, carbon dioxide, and energy.
D)ethers.
E)ketones.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
43
The dehydration product of 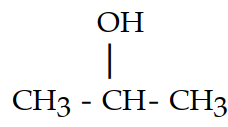 in the presence of acid is
in the presence of acid is
A)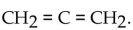
B) propene.
C) cyclopropane.
D) cyclopropene.
E) propyne.
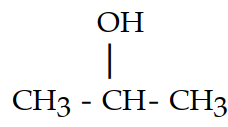 in the presence of acid is
in the presence of acid isA)
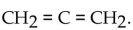
B) propene.
C) cyclopropane.
D) cyclopropene.
E) propyne.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
44
In the dehydration of an alcohol to an alkene, what is produced in addition to the alkene?
A)carbon monoxide
B)hydrogen
C)water
D)oxygen
E)carbon dioxide
A)carbon monoxide
B)hydrogen
C)water
D)oxygen
E)carbon dioxide

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
45
When a primary alcohol is completely oxidized, the product is
A)a carboxylic acid.
B)a ketone.
C)an alkane.
D)an aldehyde.
E)another alcohol.
A)a carboxylic acid.
B)a ketone.
C)an alkane.
D)an aldehyde.
E)another alcohol.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
46
Secondary alcohols are oxidized to
A)ketones.
B)esters.
C)ethers.
D)carboxylic acids.
E)aldehydes.
A)ketones.
B)esters.
C)ethers.
D)carboxylic acids.
E)aldehydes.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
47
Methanol is toxic because it is ________ by the liver to carboxylic acids.
A)conjugated
B)oxidized
C)protonated
D)hydrated
E)reduced
A)conjugated
B)oxidized
C)protonated
D)hydrated
E)reduced

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
48
Thiols can be oxidized to
A)carboxylic acids.
B)disulfides.
C)ketones.
D)thioethers.
E)aldehydes.
A)carboxylic acids.
B)disulfides.
C)ketones.
D)thioethers.
E)aldehydes.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck
49
Tertiary alcohols are oxidized to
A)carboxylic acids.
B)secondary alcohols.
C)aldehydes.
D)ketones.
E)Tertiary alcohols do not undergo oxidation.
A)carboxylic acids.
B)secondary alcohols.
C)aldehydes.
D)ketones.
E)Tertiary alcohols do not undergo oxidation.

Unlock Deck
Unlock for access to all 49 flashcards in this deck.
Unlock Deck
k this deck


